The one-two punch: giving cancer patients the dual benefit of a full-blown attack on the tumor orchestrated by their own immune system in combination with a chemical attack targeting he cancer cells.
To kick off this series of posts covering some of the science performed in the Centre for Blood Research (CBR), I will start off with my own work in Dr. Grant Mauk’s lab. His lab has a long history of work with heme proteins, which are proteins with a heme group. Heme groups are particular structures carrying iron, which gives heme proteins some very specific abilities. Hemoglobin for example, is a heme protein whose heme group allows hemoglobin to carry oxygen and thus enables the blood to transport oxygen throughout the body.
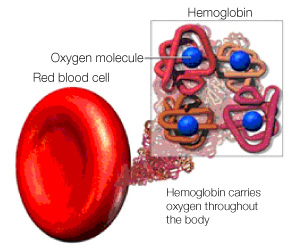
Four heme groups within each hemoglobin molecule carry oxygen throughout the body. Printed with permission from ©Masimo Corporation 2012.
Another – slightly less famous – heme protein is indoleamine-2,3-dioxygenase (IDO). IDO is an enzyme that uses its heme group to catabolize the amino acid tryptophan. For many years we thought that the sole purpose of IDO was to degrade tryptophan, but in 1998 Drs. David H. Munn and Andrew L. Mellor discovered that IDO also plays a part in regulating the immune system. High concentrations of IDO were found in the human placenta, and researchers discovered that IDO suppressed the local immune system in the placenta, and thus protected the fetus from the T-cells of the mother’s immune system.
 They further discovered that the molecule 1-methyl-tryptophan (which looks a lot like tryptophan) inhibited IDO activity and thereby removed the protective force field around the fetus. As a result, the once protected fetus was now recognized as foreign by the immune system, and the fetus was rejected from the (mouse-) mother (Munn, Zhou et al. 1998).
They further discovered that the molecule 1-methyl-tryptophan (which looks a lot like tryptophan) inhibited IDO activity and thereby removed the protective force field around the fetus. As a result, the once protected fetus was now recognized as foreign by the immune system, and the fetus was rejected from the (mouse-) mother (Munn, Zhou et al. 1998).
That IDO was able to protect a fetus from the T-cells of the mother’s immune system quickly led to the discovery that IDO also regulated the immune system in other – less favorable – situations. Many cancers, for example, suppress the immune system by making and distributing IDO to their immediate surroundings. They thereby create an IDO-mediated force field around the tumor protecting the cancer cells from T-cell attacks, and giving the tumor peace and quiet to grow and spread unnoticed (Uyttenhove, Pilotte et al. 2003).
The idea of inhibiting IDO to remove the force field, and thereby help the immune system spot and attack cancer cells, was formulated in 2003 (Uyttenhove, Pilotte et al. 2003). In parallel to removing the IDO force field from the placenta, resulting in an attack on the fetus, inhibiting IDO in cancer patients allowed a T-cell attack on the cancer cells. I.e. eliminating the force field enables the immune system to recognize the tumor and kill the cancer.
Assisting the immune system in recognizing and attacking cancer cells is called immune therapy, and immune therapy involving inhibition of IDO is becoming an increasingly hot topic. Many IDO inhibitors of varying strengths have been identified (several in Dr. Mauk’s lab), and the therapeutic potential is promising. However, although 1-methyl-tryptophan has several characteristics making it less desirable as a cancer drug, it is still the most well-studied of all IDO inhibitors.
In a joint effort with Dr. Raymond Andersen’s lab in Department of Chemistry at UBC, we are trying to identify new, better, and safer inhibitors of IDO. The Andersen team specializes in diving deep into the oceans around British Columbia and Papua New Guinea to collect a library of extracts from sponge and bacteria. In Dr. Mauk’s laboratory we examine the ability of these extracts to inhibit IDO in a test in the laboratory, and the Andersen team then isolates the active molecules in our positive hits. In this way, we have identified many novel IDO inhibitors and several brand new molecules have been discovered.
Our latest project has revealed a molecular scaffold (a so-called pharmacophore) that is capable of inhibiting IDO. Adding different chemical groups to this scaffold creates new molecules with varying strengths in terms of IDO inhibition, and different degrees of side-effects in the human body.
 Once we have a candidate that shows the best potential in the laboratory, we will move into a more physiological model. And hopefully down the road, we will be able to add an IDO inhibitor to a chemotherapy treatment regimen, giving cancer patients the dual benefit of a chemical attack on the cancer cells as well as a full-blown attack orchestrated by their own immune system.
Once we have a candidate that shows the best potential in the laboratory, we will move into a more physiological model. And hopefully down the road, we will be able to add an IDO inhibitor to a chemotherapy treatment regimen, giving cancer patients the dual benefit of a chemical attack on the cancer cells as well as a full-blown attack orchestrated by their own immune system.
Anne Steino.
(References:
Munn, D. H., M. Zhou, J. T. Attwood, I. Bondarev, S. J. Conway, B. Marshall, C. Brown and A. L. Mellor (1998). “Prevention of allogeneic fetal rejection by tryptophan catabolism.” Science 281(5380): 1191-1193.
Uyttenhove, C., L. Pilotte, I. Theate, V. Stroobant, D. Colau, N. Parmentier, T. Boon and B. J. Van den Eynde (2003). “Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase.” Nat Med 9(10): 1269-1274.)