Recently, Dr. Chris Orvig and his research group from the UBC Chemistry department published “H2hox: Dual-Channel Oxine-Derived Acyclic Chelating Ligand for 68Ga Radiopharmaceuticals” in Inorganic Chemistry on February 18, 2019. This involves the development of a new ligand, H2hox, for potential use in radiolabelling of cancer cells within the body. The video below describes the radiolabelling process and why it is useful (warning: footage of a surgery from 4:08 onwards):
In general, ligands are compounds that bind to metal ions. These compounds can be positively charged, negatively charged, or neutral. Ligands that bind to a metal through multiple sites are known as polydentate ligands and tend to be more stable. H2hox is a hexadentate ligand, which means it can bind to 68Ga at six different sites.
A main portion of ligand design is to prevent radioactive metal ions from being attracted to negative phosphate ions in bone. On the use of gallium, Dr. Orvig explained “68Ga has several useful radioactive properties, and many ligands to bind with it have been developed. However, those existing ligands have different degrees of limitations.” One such example given in this paper is the commonly used ligand DOTA, which requires longer time until imaging can occur and high temperatures. This presents a problem in living beings as high temperature is harmful.
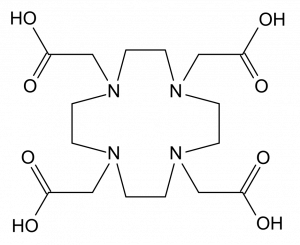
The structure of DOTA. Source: Wikimedia Commons
H2hox, the centerpiece of this new publication by the Orvig research group, has many advantages over other existing ligands that can bind to 68Ga. For one, the synthesis of H2hox is easier and faster than other previously reported ligands. H2hox tightly binds to 68Ga, and rapidly achieves efficient and reproducible radiolabeling in living systems (in vivo). These properties mean H2hox is applicable to cancer diagnosis and radiotherapy treatment for cancer.
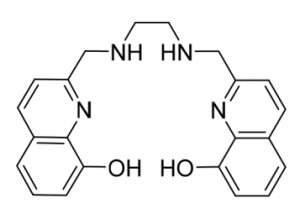
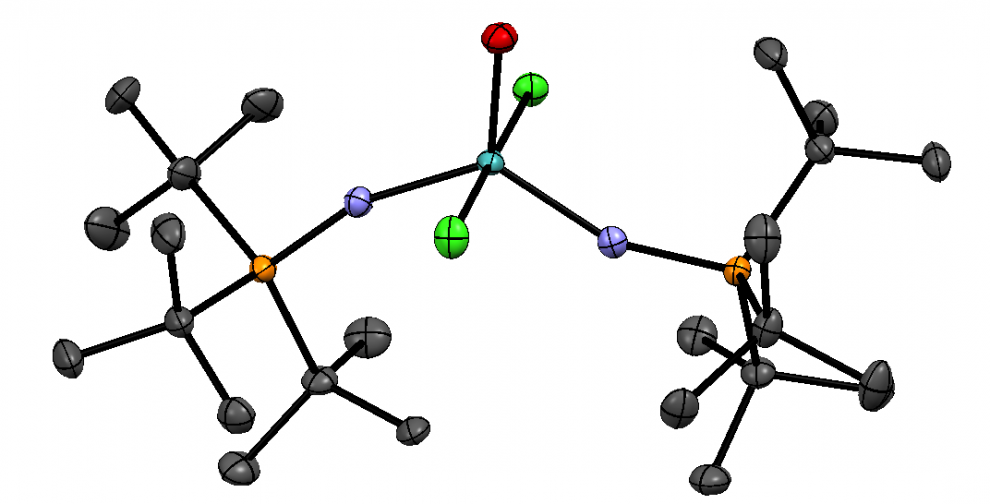
The structure of H2hox. Source: Wang et al., 2018
“We have looked at [H2hox] in mice, and we have successors of that ligand that work with bigger metal ions that also work in mice very well,” said Orvig. Given all the tests on in vivo systems succeeded, Orvig replied that “The idea would be that we could potentially use derivatives of hox that are functionalized for positron emission tomography (PET). We view hox mostly as a potentially diagnostic ligand since it and the metals it coordinates to are small.” On what’s next for hox, Dr. Orvig said, “The next step now is to add the targeting vector (helps target cancer cells), and we’re working on that right now, then we would test that on a tumor model with the BC Cancer Agency. If all of that works then we might talk about going to a higher animal model.”
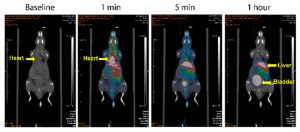
PET images of Ga-hox in mice. Source: Wang et al., 2018
Here is a video giving a brief introduction to positron emission tomography:
“This paper is a new kind of ligand because it contains oxinate (oxine bonded to metal) moieties, because oxinates bond very, very strongly to metal ions,” said Dr. Orvig. This helps prevent the gallium separating from H2hox and depositing in the body. “We are trying to create a ligand that could be useful for gallium, hence the focus in the paper, or copper because both gallium and copper are potentially useful for cancer radiotherapy,” Dr. Orvig explained.
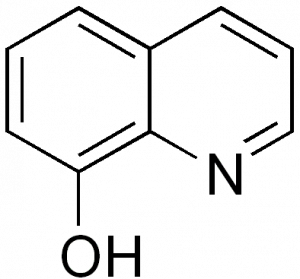
Caption: The structure of oxine. Two of this structure can be observed in the H2hox image above. Source: Wikimedia Commons
According to Dr. Orvig, this research is intended to help with cancer treatment and making the process more consistent. “Chemotherapy essentially beats up every cell in the body, but the cancer is a little more susceptible since it’s dividing faster. What we’re trying to do is target radioactive isotopes to the cancer so that you can both specifically detect it and then specifically treat it with different forms of radiation. If you can specifically target it, then you can deal a lot more damage to the cancer cells than the healthy cells and avoid chemically poisoning chemotherapy patients.” This video explains the concepts behind chemotherapy:
https://www.youtube.com/watch?v=wjaFhdOowAg
Dr. Orvig’s group is pushing forward on the development of targeting vectors as part of a large government funded project that involves collaborations with co-investigators. Through a team effort, scientists at TRIUMF develop and produce the required gallium radioisotopes, while researchers at the BC Cancer Agency are working with Dr. Orvig’s group on testing cancer models. Specific cancer types that the BC Cancer Agency uses as a starting point are prostate cancer and a form of breast cancer, which are two common forms of cancer that are responsible for roughly 9100 deaths in Canada every year. These models are transplanted onto mice and tested.
While this new ligand system is an important advance in the area of radiopharmaceutical drug development, Dr. Orvig was careful to note that this is not a panacea for all types of cancer. “I often liken cancer to the common cold. The common cold is caused by some 200 different viruses, so the common cold is essentially 200 different diseases. Cancer is also a collection of different diseases. What they have in common and why they’re cancers is because the cells are dividing more rapidly than healthy cells and in a toxic fashion. So I would definitely not say H2hox is useful for a broad spectrum but for some very specific applications.”
~ Nicholas Patterson, Danial Yazdan, Wenxin Zhao, Samuel Gong