As you may have already realized, living in Vancouver means it’s gloomy, cold, and wet 10 out of the 12 months each year. As a Vancouverite, an umbrella is an essential item to take with you when leaving your house. But imagine, one day you’re walking down the street and a giant, sparkling diamond falls from the sky and lands right before you. How is this possible? Is it… raining diamonds?!
This is quite impossible on planet Earth, however, it may be the case for our gas giants, Jupiter, Saturn, and ice giants, Uranus and Neptune. Study shows that the atmosphere of these distant planets are filled with methane gas, a gas molecule with the molecular formula CH4, and have the ideal conditions for diamond formation. However, the proportions of methane gas in Jupiter’s and Saturn’s atmosphere is significantly lower than Uranus’s and Neptune’s atmosphere.
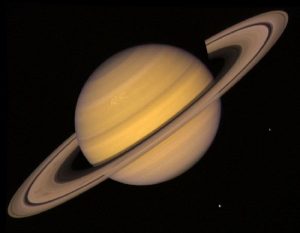
Saturn Source: Flickr
Despite this difference in methane availability, spacecraft Cassini has reported sightings of lightning storms in the upper atmospheric region of Saturn. The presence of lightning can break up methane gas into soot (the carbon source for diamonds); similar storms are apparent on Jupiter. As soot sinks deeper into the atmosphere, pressure also increases. The increase in pressure converts soot into graphite. Once graphite is made, it continues to fall deeper and deeper towards the planet’s core; with increasing temperature and pressure, diamonds are formed!
However, as the solid diamonds fall closer to the core of Jupiter and Saturn, the temperature also increases dramatically to approximately 12 000⁰K. This temperature is well above the melting point of diamonds, approximately 3800⁰K, thus creating a sea of diamond liquid. Albeit, the temperature on Uranus and Neptune is much cooler in comparison. As a result, diamonds formed in these planets stay in their solid state.

Diamond in its solid state Source: Flickr
The possible size of these diamonds formed also vary. Just like rain, when the diamonds fall deeper into the atmosphere, they are capable of growth. When falling, some of the precious gems can grow so large that they can be classified as “diamond-bergs” (the size of an iceberg!).
Before you get all excited and wowed by this, I must say that is indeed not a fact. Unfortunately, this is only a theory from NASA and their scientists from observing the conditions and properties of these planets. We can only infer since we are restricted with the ability to travel to either Jupiter, Saturn, Uranus or Neptune to formally further investigate this research topic. Perhaps one day, technology will be advanced enough for us to send robots, or maybe even humans farther than Mars and investigate extraterrestrial phenomena and their precious secrets held within.
A video of how diamonds are formed on Earth:
-Katrina Lim
Resources:
- National Geographic. Diamonds stud the atmospheres of Saturn and Jupiter. http://news.nationalgeographic.com/news/2013/10/131009-diamonds-saturn-jupiter-planet-science-space/ (accessed Nov. 5th 2016)
- Cassini: unlocking Saturn’s secrets. http://www.nasa.gov/mission_pages/cassini/whycassini/planet.html (accessed Nov 5th 2016)