Some people feel like they are always stuck to the outlet because our current batteries cannot hold enough power and degrade quickly. (c) JuralMin, released under Creative Commons CC0
Have you ever been in a situation where you need to charge your phone multiples times a day in order to maintain its battery level? We’ve all probably experienced times when we desperately need to use our phones but the battery is completely drained, with only moderate use throughout the day. Why is it that current cellular device technology has improved significantly, but battery quality has not?
Recently, researchers at the University of Cambridge have developed a new type of battery that may soon solve this problem and increase the productivity and lifespan of the modern lithium-ion battery. This new type of battery, called the lithium-sulfur battery, takes inspiration from the villi in our digestive intestinal tract. It is predicted that these next-generation batteries could potentially hold up to five times the energy that a tradition lithium-ion battery can, without increasing the size of the battery.
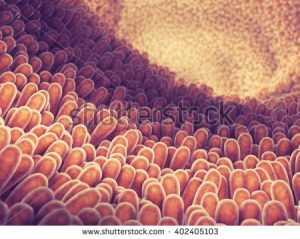
A illustration of what villi look like in the small intestine (c) nobeatsofierce
Villi are finger-like protrusions lining the intestinal tract which increase the surface area needed for the absorption of nutrients. In the battery, zinc oxide wires stick out like villi from the surface of one of the battery’s electrodes, the part of the battery that generates electrons.
In traditional lithium-ion batteries, active material in the battery that break off the flat electrode are lost and lead to the degradation of the battery. This new villus configuration allows precious “battery nutrients” that break off the electrode to be attracted to the villi wires, just like how the villi in the intestines attract and absorb the nutrients on our food. The material attracted by the zinc oxide wires can then be reused for the production of electrochemical energy, greatly increasing the lifespan of the battery.
The lithium-sulfur battery also has a higher energy density than regular lithium-ion batteries due to the ability of sulfur to hold a higher number of lithium-ions than carbon can in the current battery model. To illustrate this, imagine that the battery is a factory and lithium ions are the workers. The carbon factory only has enough capacity for 100 workers. However, the sulfur factory can have >500 workers. Which factory will be more productive? This is why carbon is replaced by sulfur in the new-type battery, due to sulfur’s ability to hold more lithium-ion “workers” to generate more energy.
These breakthrough changes, substituting carbon for sulfur and the addition of zinc oxide “villi”, result in a new type of battery that surpasses the lithium-ion battery in terms of energy capacity as well as battery lifespan.
“By taking our inspiration from the natural world, we were able to come up with a solution that we hope will accelerate the development of next-generation batteries.” said the study’s lead author Teng Zhao.
However, the battery is currently not yet ready for commercial use and will probably not be available without further research and development.
The new advances in battery technology is exciting and brings hope to all of us who use battery-powered devices. I think it’s about time that the batteries we commonly use now receive an upgrade. Even though this new technology has not yet been perfected, I believe that through the discovery of lithium-sulfur batteries we can move towards developing better batteries for the future.
-Charlie Wei
References:
University of Cambridge. “Next-generation smartphone battery inspired by the gut.” ScienceDaily. ScienceDaily, 26 October 2016. <www.sciencedaily.com/releases/2016/10/161026102701.htm>.