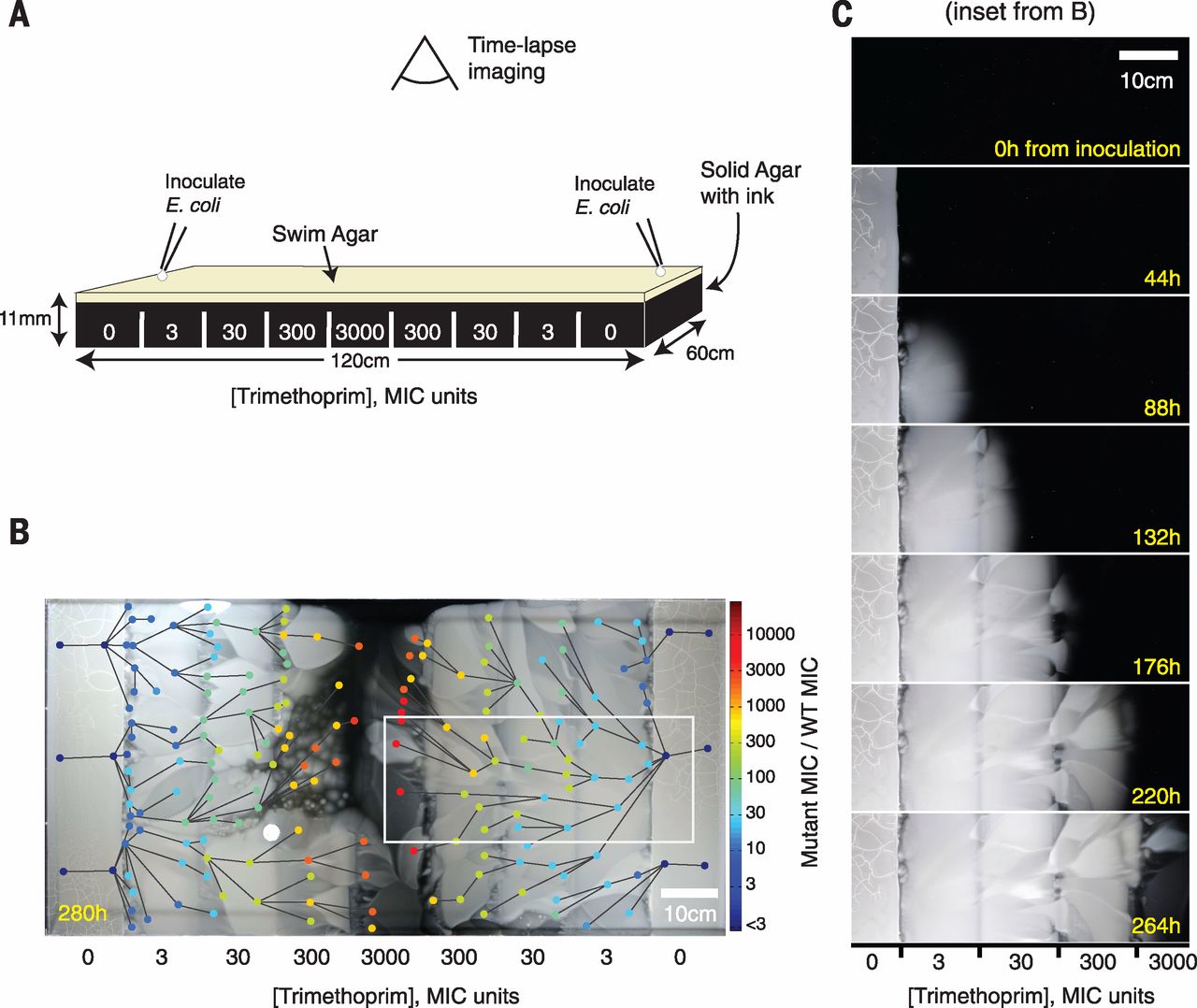
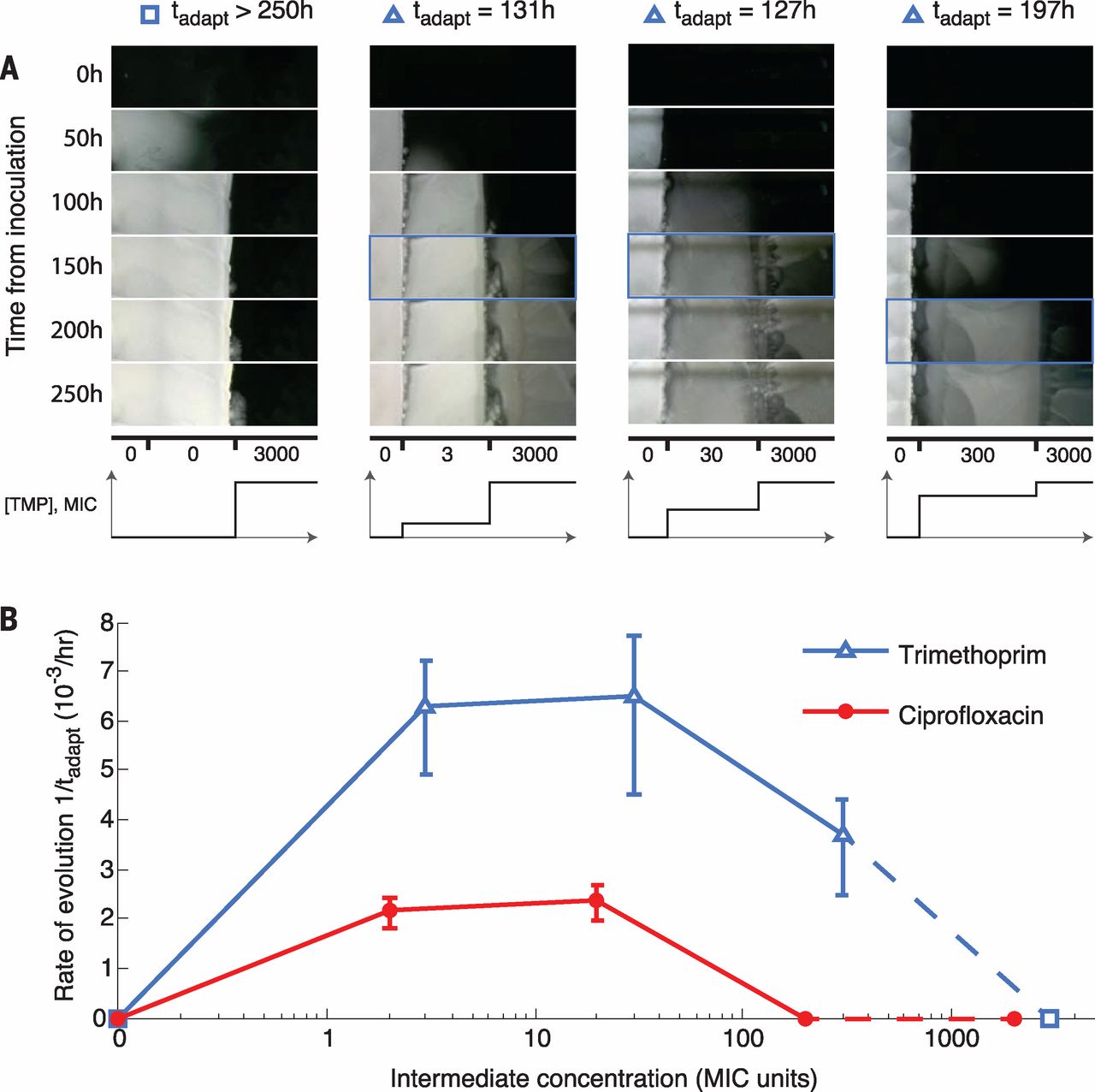
Aluminum was reported to be capable for removing mineral in water without involving sodium, according to online Environmental Science & Technology on Oct 4. This new method was motivated because of the negative effect resulted by current sodium-related water soften method, says by study coauthor Arup SenGupta.
As an environmental engineer at Lehigh University in Bethlehem Pa, Sengupta believes that improving the traditional way of softening water can bring benefits to environment.
Hard water, which is defined as water contains significant amount of mineral ion such like calcium and magnesium , is relevantly difficult to lather by soap and usually form incrustation in containers or tubes.
Introduction to hard and soft water: 
The current method for solving problem of hard water is filtering through a tank with glass beads. The sodium ions covering on beads can exchange with mineral ions ,and this leads to soft water.
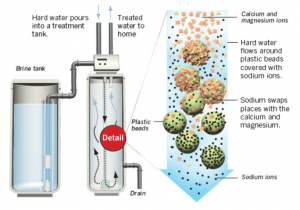
However, this method has many negative effects. On one hand, the sodium added has negative effect on people’s health, for example, they can raise blood pressure. On the other hand, the system can not solve the problem once and for all, since it needs to be recharged by sodium-based brine regularly.
From the point view of chemistry, SenGupta and his colleagues select aluminum as a substitute of sodium. Actually aluminum ion has three positive charges and that means it is relatively unlikely for aluminum ion to exchange with mineral ions. So why they choose aluminum ion? The reason is that the aluminum can precipitate and gather on beads after exchanging instead of being taken away like sodium. This makes aluminum ions reusable.
The new system of aluminum was tested by researchers’ lab and was found to have better performances than old systems. It’s worth mentioning that the set up used was similar and that can make the possible renewing project in the future easier and cheaper.
So far, this new technique will still be challenged in practical use. “I see these great things all the time, but a lot of them just don’t make it financially,” said by Steven Duranceau, an environmental engineer at the University of Central Florida in Orlando. On contrary, SenGupta keeps his enthusiasm, as he says “This is not a magic bullet; there are shortcomings, but none of these problems are impossible to overcome.”
Reference
J. Li et al. Aluminum-cycle ion exchange process for hardness removal: a new approach for sustainable softening. Environmental Science & Technology. Published online October 4, 2016. doi: 10.1021/acs.est.6b03021.