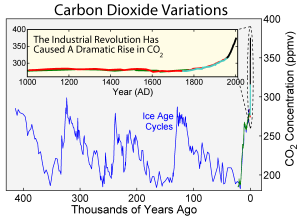
A graph showing the rise and fall of CO2 concentration in the atmosphere, recorded over thousands of years. The magnified area indicates the dramatic increase of CO2 concentration during the Industrial Revolution. (c) Robert A. Rohde, used under Creative Commons Attribution-Share Alike 3.0 Unported
There is no doubt that climate change is real, dangerous, and occurring at an alarming rate that is unprecedented in the past 1,300 years. A major of the cause of this change is due to carbon dioxide gas, the product of burning fossil fuels for energy to run our cars, factories, for the production of electricity, and more. Carbon dioxide, one of many greenhouse gas, naturally acts as sort of a “blanket”, absorbing and emitting infrared radiation from the earth, causing the atmosphere to warm up, which known as the greenhouse effect.
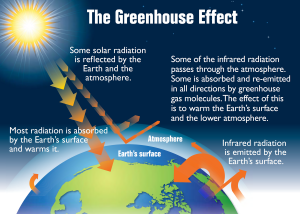
A diagram illustrating the greenhouse effect. (c) US EPA used under public domain
Initiatives to reduce carbon dioxide emissions have already been implemented in our everyday lives, for example a simple thing like biking or taking public transit can reduce the amount of carbon dioxide emitted by automobiles. However, new carbon dioxide emissions data shows that our efforts are not paying off. Every year, it is estimated that 38 billion tons of unnecessary carbon dioxide is released into the atmosphere. Even as you read this article 2.4 million pounds of this greenhouse gas is released into the atmosphere per second!
It seems that our efforts to reduce carbon dioxide emissions have failed and each year we can see a steady increase in emissions. Our economic and societal infrastructure has made us incredibly dependent on burni
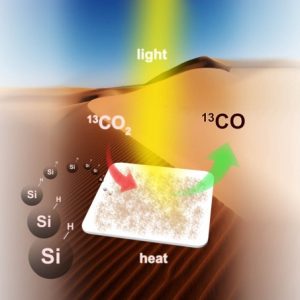
Simple illustration of the conversion of CO2 into CO using silicon. (c) Chenxi Qian, used under Creative Commons Attribution 4.0 International License.
ng fossil fuels for energy. Perhaps the real solution lies in taking the excess carbon dioxide gas and converting it back into usable energy.
Recently, scientists from the University of Toronto believe to have discovered a method of converting carbon dioxide gas into energy-rich fuel. Professor Geoffrey Ozin and his team have developed a method using silicon, naturally found in sand, to efficiently and selectively convert gaseous carbon dioxide to carbon monoxide without any harmful emissions. Carbon monoxide can then be converted into hydrocarbon fuels such as petrol through a series of chemical reactions known as the Fischer-Tropsch process.
“A chemistry solution to climate change requires a material that is a highly active and selective catalyst to enable the conversion of CO₂ to fuel. It also needs to be made of elements that are low cost, non-toxic and readily available,” said Dr. Ozin.
Right now they are working on ways to increase the activity, enhance the scale, and boost the rate of production. Hopefully in the near future, there will be even more research dedicated to converting other greenhouse gases, not just carbon dioxide, into reusable energy, and then maybe we will be able to resolve the issues that have been caused by the detrimental amounts of greenhouse gases in our atmosphere.
– Charlie Wei