Monday, November 7, 2016. The standard time has returned and we gained an hour of our time back. It is nice to sleep in and have one more hour of sleep, but have you wondered why we do this? George Hudson first proposed Daylight Saving Time (DST) a century ago and countries adapted this system to save energy (1). Now you must be wondering: how does changing the time on a clock save energy?
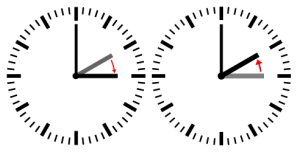
Figure 1: The clock is moved forward an hour (left) in March. It is called the Daylight Saving Time (DST). When the time is moved back an hour (right) in November, it is called the Standard Time. Photos and edits by Daniel FR, Plenz (Original by Daniel FR, SVG by Plenz) [Public domain], via Wikimedia Commons

It turns out that this question is very hard to answer. Many scientists disagree with each other: some say it does and some say it does not. However, the consensus is that the effects of DST on electricity usage is minimal (0.5-2%) (2). Scientists also concluded that DST also does not encourage physical activities (3) in any way. Clearly, DST is not serving its purpose; however, there is something that scientists suspect that DST may be responsible for.
According to a study conducted in 2014, the day following the DST in the spring (the clock is moved forward and we lose an hour of our time), the number of heart attacks increased by 24% (4). Vice versa, when the clock was moved backward and we gained an hour of our time, the number of heart attacks decreased by 21% (4). The hours of sleep and sleep quality are deeply correlated with heart attacks (5) and scientists suspect suddenly losing an hour of our sleep in the spring caused this increase. On the other hand, gaining an hour of sleep is thought to be decrease the number of heart attacks after DST in fall. Many other studies came to the same conclusion (6, 7) that DST may be influencing the number of heart attacks.
Daylight Saving Time (DST) seems like another ridiculous tradition that just does not work any more for our modern society. Its ability to decrease energy consumption is questionable and it may put people at higher risks of heart attacks. Plus, no one needs more confusion calculating the time difference between time zones. Although I love gaining one hour of sleep in the fall, it’s clear it is time to ditch DST.
-Jay Park
REFERENCE
1. CGP Grey,. Daylight Saving Time Explained https://www.youtube.com/watch?v=84aWtseb2-4&t=191s (accessed Nov 7, 2016).
2. Aries, M.Newsham, G. Effect of daylight saving time on lighting energy use: A literature review http://www.sciencedirect.com/science/article/pii/S0301421507002273 (accessed Nov 7, 2016).
3. Zick, C. Does Daylight Savings Time Encourage Physical Activity?: Journal of Physical Activity and Health: Vol 11, No 5 http://journals.humankinetics.com/doi/10.1123/jpah.2012-0300 (accessed Nov 7, 2016).
4. Sandhu, A.; Seth, M.; Gurm, H. Daylight savings time and myocardial infarction (accessed Nov 7, 2016).
5. Andrechuk, C. Ceolim, M. Sleep quality and adverse outcomes for patients with acute myocardial infarction J Clin Nurs 2015, 25, 223-230.
6. Kirchberger, I.; Wolf, K.; Heier, M.; Kuch, B.; von Scheidt, W.; Peters, A.; Meisinger, C. BMC Public Health 2015, 15.
(7) Toro, W.; Tigre, R.; Sampaio, B. Daylight Saving Time and incidence of myocardial infarction: Evidence from a regression discontinuity design. Economics Letters 2015, 136, 1-4.