What would happen if we ran out of antibiotics to use against bacteria? Antibiotic resistance is known to be one of the major concerns among doctors and scientists around the world since, it’s our primary defense against bacterial infections. Without these antibiotics, it’d be virtually impossible to treat many types of diseases or even perform surgeries.
The quality of healthcare has significantly improved over the centuries as more people have access to treatment, medicine, and antibiotics. However, it has also increased the risk of wrong applications of drugs, allowing the bacteria to develop defense mechanisms to different types of antibiotics. In fact, there has been a dramatic increase in the number of scientific reports about different drugs resistance cases.
Antibiotic resistance occurs through process known as ‘Natural Selection’, where the bacteria has evolved and is no longer affected by the antibiotics. A stand-out candidate as an alternative for antibiotics is known as bacteriophages.
‘Alternative treatments are urgently required and we are investigating one such treatment – the use of bacteriophage.’
- Robert Atterbury, Phage Biotechnology, University of Nottingham.
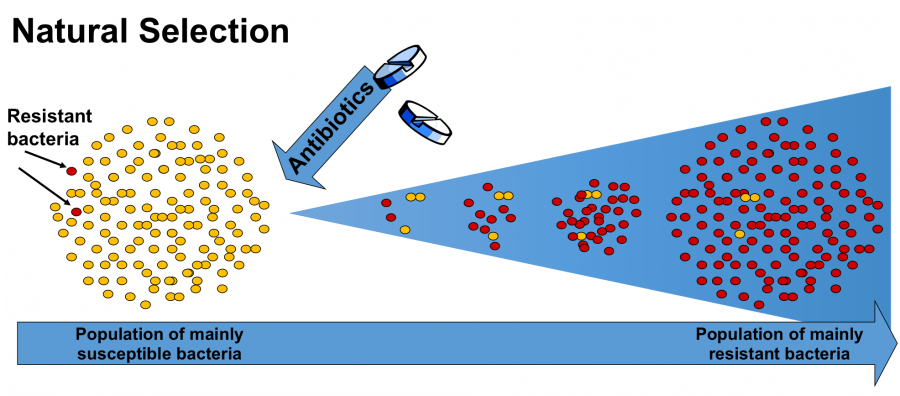
Resistance bacteria survived the treatment of antibiotics, thus able to reproduce and increase in numbers. <http://www.reactgroup.org/toolbox/mutation-and-selection/>. Photo credit: Uppsala University
‘Bacteriophage, or phage is a virus that infects bacteria, so these don’t infect human cells- they are specialised and only infect bacteria’
- Brent Gilpin, Science Leader, Environmental Science Group, New Zealand.
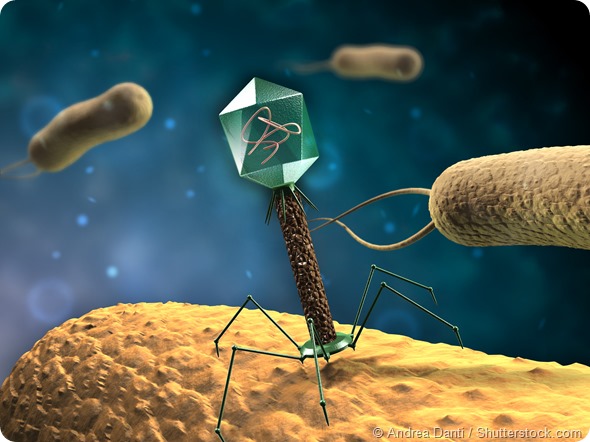
Bacteriophage attached itself to the bacteria before releasing its DNA inside. <http://www.news-medical.net/news/20151202/Bacteriophage-therapy-an-alternative-to-antibiotics-An-interview-Professor-Clokie.aspx>. Photo credit: News-Medical.net team
Bacteriophage or phage infects the bacterial cell by first recognizing the bacteria and then attaching itself to the bacteria’s surface (cell wall). After the phage has penetrated the cell wall, entering the bacteria, and it releases its DNA inside. This DNA merges itself with the bacterial DNA causing the bacteria to produce proteins for the phage. Other chain reactions occur which causes the bacteria cell to produce more phages and eventually bust out, causing the bacteria to die. – This process is shown in the following video:
This method is currently being adopted by many industries including food protection against food-borne disease, and medical treatment for both animal and humans. Furthermore, there are many advantages in using phage therapy; for example the phages are target specific, thus only attacking bacteria with a certain structure. With the right phage, it’s harmless to humans and since, phage is found naturally throughout the environment, there are several types of phages that can be studied and used. In addition to this, phage can be genetically modified to reduce their side effects, harmful abilities, or any unnecessary features. With this, it’s possible that phages can be used as an efficient and effective treatment against bacteria.
I strongly believe that bacteriophage is a potential alternative for antibiotics due to its ability to target specific bacteria, its harmless nature to humans, and its ability to be genetically engineered. Moreover, it can also be used in many industries as a safety precaution, in medical treatments, or even scientific research.
Poramat Sucharit