Halloween has approached us. Among the daunting horror films and scary costumes come the dreaded likelihood of cavities AKA dental caries (DUN DUN DUN)!

Image Credit: Cartoon Stock https://www.cartoonstock.com/directory/c/cavities.asp
As a child I always wondered how cavities form, but I never looked into it because I didn’t want to face the awful truth that my beloved candy is actually plotting against me. But now that I am older, and hopefully wiser, I have figured out the true story of the candy cavity curse!
It begins the moment you present that delicious piece of candy to your mouth, and the simple sugars, specifically sucrose, are released into your oral cavity. The sugar feeds the already present bacteria on your teeth [1], which live in the plaque growing on the enamel. Plaque forms normally on your teeth by the combination of sucrose, and proteins from your saliva [2]. However unpreventable, it can be removed by frequent tooth-brushing. So bacteria such as Streptococcus mutans (S. mutans), which are round, cariogenic (causes tooth decay), and anaerobic, feed on the sucrose provided by the candy, to form lactic acid through glycolysis [2].
Glycolysis is an important process in which energy is produced. The process is outlined generally below.
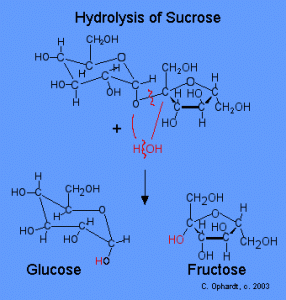
Hydrolysis of Sucrose to form Glucose and Fructose Image Credit: Charles E Ophardt http://chemistry.elmhurst.edu/vchembook/548toothdecay.html
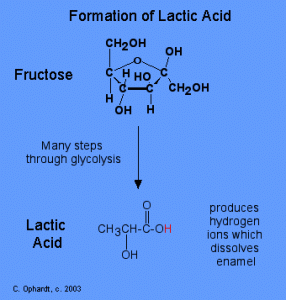
Formation of Lactic Acid from Fructose via Glycolysis. Image Credit: Charles E Ophardt http://chemistry.elmhurst.edu/vchembook/548toothdecay.html
S. mutans lives in the plaque trapped between your teeth, and lacks oxygen. Therefore it uses glycolysis to produce lactic acid under anaerobic conditions [3]. Lactic acid is very acidic. It has a pH level of 2 [4]. The added acidity decreases the pH of your mouth to initiate the dissolution of the calcium phosphate in your tooth enamel [3]. Thus, the start of a cavity. A description of the process is explained in the video below.

Despite the terror inflicted on my teeth due to my obsession with candy, I refuse to deprive myself of the traditional Halloween treat. Although ceasing the intake of sugar would obviously decrease the production of lactic acid on my teeth, also decreasing cavity formation, there are other methods being investigated to protect our teeth.
For example, Shelby Kashket, and Dominick P. DePaola have studied the anticariogenic effects of cheese [5]! They researched that possibly due to the buffering effects of dairy proteins on the lactic acid formation, and increased salivation when eating cheese, this helps in battling the cariogenic effects of S. mutans!
Additionally, Chu-hong Hu et al. developed a way to make large quantities of Glycyrrhizol A, an extraction of licorice root, into a sugar-free lollipop which can kill the S. mutans bacteria [6]! Talk about fighting fire with fire, or in this case, fighting candy with candy!
Finally, you can also use toothpaste with sodium bicarbonate, which raises the pH level in your mouth and neutralizes the acid [1]. Or rinse with fluoride, which speeds up the remineralization of the enamel, too [7].
So, I guess what I’m saying is, long live candy! And, apparently, cheese!
…Just make sure you brush and floss often, as well.
Happy Halloween, everyone!
Nicole Yipp
References:
[1] Dr Chemical, http://drchemical.com.au/why-does-sugar-cause-tooth-decay (accessed Oct 31, 2016).
[2]Loesche WJ. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986;50:353–380.
[3] Ophardt, C. E. Tooth Decay http://chemistry.elmhurst.edu/vchembook/548toothdecay.html (accessed Oct 31, 2016).
[4] Lactic acid; MSDS No. 9924447 [Online]; Science Lab; Houston, Texas, May 21, 2013, http://www.sciencelab.com/msds.php?msdsId=9924447 (accessed Oct 31, 2016).
[5] Kashket, S.; Depaola, D. P. Nutrition Reviews 2002, 60 (4), 97–103.
[6] Hu, C. H.; He, J.; Eckert, R.; Wu, X. Y.; Li, L. N.; Tian, Y.; Lux, R.; Shuffer, J. A.; Gelman, F.; Mentes, J.; Spackman, S.; Bauer, J.; Anderson, M. H.; Shi, W. Y. International Journal of Oral Science 2011, 3 (1), 13–20.
[7] Fluoride And Your Teeth http://www.colgate.com/en/us/oc/oral-health/basics/fluoride/article/fluoride-and-your-teeth (accessed Oct 31, 2016).
