Plastic is an material that we all use on a daily basis, sometimes without thinking about it. Our food and drink come stored in plastic containers, our toiletries do too. our clothes are sometimes made of plastic and our cars contain plastic parts. Plastic is highly useful because it is easy to produce, cheap to buy, and usually lasts a long time. After we have finished using the plastic, what becomes of it? Where does it go? Sometimes we recycle it, but not often enough. And often, it ends up in the ocean, causing harm to plants, animals and humans.

Photo: https://www.chinadialogue.net/culture/10123-Plastics-are-making-our-oceans-sick/en
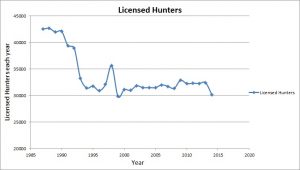
The world produces about 300 million tonnes of plastic every year. Some of that plastic is used for long periods of time, such in car parts, while other forms of plastic are used only briefly, such as disposable drink bottles. When all this plastic is disposed of, some of it gets recycled, some sent to landfills, and some incinerated. Still more of it finds its way into our oceans. It has been estimated that this amount is between 4.8 and 12.7 million tonnes every year, a number which continues to increase.

Photo: http://www.takepart.com/article/2014/12/10/worlds-ocean-plastic-pollution-problem-just-got-bigger–lot-bigger
But once the plastic is in the ocean, it doesn’t just disappear. It has to break down into smaller chemical particles over time, which, depending on the type of plastic and the environment of degradation, can be as short as 50 days or as long as a thousand years. Often the plastic lifetimes are on the larger side of this range. Within that time, the plastics can be ingested by animals, often resulting in their death, which affects animals populations including our food sources such as fish. The plastic can also get caught on their bodies, or washed up on beaches, or slowly degrade and release harmful toxins into the water.
Despite being useful, plastics pose problems to the environment. They have already done damage, and we need to deal with the problems that have arisen, while preventing the situation from growing worse, while ensuring that we look after our planet. Plastic in the ocean is a bad idea, and a large problem we have to deal with.
Nathan Heyns.