For centuries, fossil fuels have been the most common energy source in the world, and are still used extensively. However, with the growing problem of climate change due to climbing levels of carbon dioxide in the atmosphere, combined with the prospect of limited sources of fossil fuels available, interest in renewable and more environmentally friendly sources of energy is growing.
Sunlight is a well-known source of natural energy, and can be converted into usable energy sources. Probably the most well-known method is the use of solar cells to produce electricity from sunlight, as cells have been in use for decades, in applications ranging from satellites to calculators. Today, research in renewable energy continues, as scientists investigate the possibilities of using sunlight to produce other usable energy sources besides electricity.
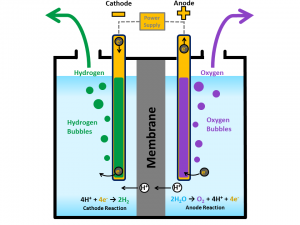
In photosynthesis, water is split into hydrogen and oxygen, which are combined with carbon dioxide to build biochemical molecules. Within the last half a century, laboratory processes have replicated the splitting, imitating photosynthesis in plants. The hydrogen can be collected and used immediately as a fuel, e.g. in rocket engines, or used to make other fuels. The most basic of such methods involves using electricity passed between two electrodes in water, producing hydrogen and oxygen gas at the electrodes, as shown below(1). This method does not use sunlight directly, but the electricity could be supplied from solar cells. An alternative method of water splitting uses a solar cell containing a Titanium Oxide (TiO2) electrode to absorb sunlight and produce hydrogen and oxygen on the cell surface. However, the energy conversion of this method is highly inefficient, and is therefore not widely used (2).
Another approach to artificial photosynthesis involves a closer imitation of its biological analogue, using both water and carbon dioxide to produce fuels. One known method, also using a TiO2 catalyst, converts water and CO2 to oxygen and small hydrocarbon molecules including methane and methanol. Both of these are common fuels. The Titanium catalyst can be modified with elements such as copper, platinum or silicon to make the production of hydrocarbons more selective. For example, adding platinum to the catalyst increases the preference of methane over methanol eight times higher than before (3). Despite the useful carbon compounds produced in these process, carbon monoxide (CO), a toxic gas, is also produced. However, the amount of CO produced can be decreased by further catalyst modification, but not entirely eliminated.
These catalytic methods are beneficial in producing useful fuels from clean and renewable energy sources and consume a greenhouse gas (CO2) in the process. This provides a favourable solution to both climate change as well as an energy crisis. The downsides of these methods are the expense and inefficiency, and thus are not yet used in mass production of fuels.
References:
- https://energy.gov/eere/fuelcells/hydrogen-production-electrolysis. (Accessed 08/02/2018.)
- Bard, Allen J., and Marye Anne Fox. “Artificial photosynthesis: solar splitting of water to hydrogen and oxygen.” Accounts of Chemical Research 28.3 (1995): 141-145.
- Mul, Guido et al. “Artificial photosynthesis over crystalline TiO2-based catalysts: fact or fiction?.” Journal of the American Chemical Society 132.24 (2010): 8398-8406.
