We are living in “the polymeric world”. What does it mean by the polymeric world? Look around you! We cannot keep away from the materials made up of polymers. Probably, the most common polymer exposed to our body would be a polyurethane, if you sleep on some sort of a comfortable mattress.
The use of polyurethanes
How does a mattress relate to a polyurethane? The polyurethane is a cushioning material to produce a flexible and rigid foam. More than 50 % of polyurethanes are consumed to make the foams (Figure 1). In addition to the function of recovering an original shape, the rigidity in the range between a flexible rubber and a hard thermosetting plastic makes polyurethanes the best material for mattresses.
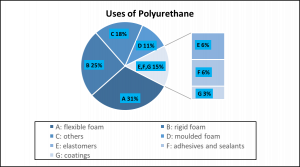
Figure 1. Uses of polyurethanes for various materials. Mainly, polyurethanes are used to produce foam materials. This figure is modified from the open source
The carbamate group and the backbone of polyurethanes
How could polyurethanes have rigid and flexible properties? The chemical structure of a polyurethane would explain its properties. The polyurethane is a block polymer produced from two monomers, a polyol and a diisocyanate. The reaction between hydroxyl and cyanate groups gives a rise to repeating carbamate groups in a long chain (Figure 2; left). The polar carbamate group can have intermolecular hydrogen bonding, resulting in the decrease of free volumes within a polymer system (Figure 2; right, Figure 3). Therefore, polyurethanes can have the rigid property associated with the carbamate group.
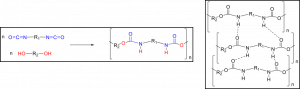 Figure 2. A chemical reaction of a diol and a diisocyanate to form a polyurethane (left). Intermolecular H-bonding of polyurethane chains (right).
Figure 2. A chemical reaction of a diol and a diisocyanate to form a polyurethane (left). Intermolecular H-bonding of polyurethane chains (right).
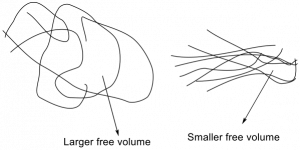 Figure 3. A flexible polymer system due to a large free volume (left). A rigid polymer system due to a small free volume (right). Polyurethanes would resemble the small free volume system due to intermolecular hydrogen bonding.
Figure 3. A flexible polymer system due to a large free volume (left). A rigid polymer system due to a small free volume (right). Polyurethanes would resemble the small free volume system due to intermolecular hydrogen bonding.
The backbones of polyol and diisocyanate are also an important factor to control the flexibility of polyurethanes. The flexibility of a long hydrocarbon chain, which both or either the monomers can have, would be introduced intrinsically to the polymers. This implies that polyurethanes can be variously derivatized, switching the backbone of diols and diisocyanates.
-Young Cho

2 responses to “Polyurethane – a chemical in your mattress”