“Organic synthesis”, seems like a mysterious area to many people without a chemistry degree. Generally, organic chemists synthesize molecules with academic values or commercial values, like drugs and catalysts. However, there are some chemists like to create some fun molecules, which are usually thought to be useless.
Dr. James Tour at Rice University is a famous chemist in building “useless” molecules, such as “NanoKid” and “NanoCar”. In April 2003, he published an article of synthesis “NanoKid”, as well as “NanoProfessionals” based on the NanoKid.
Synthesis and Modifications of the NanoKid
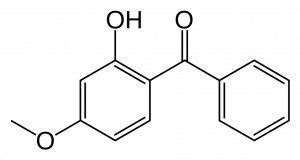
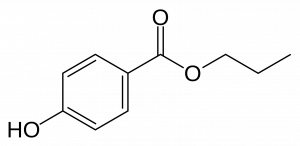
A NanoKid is formed by two parts: an upper body and a lower body. The upper and lower bodies were connected by a Pd/Cu-catalyst through a Sonogashira Reaction. Meanwhile, the “head” of a NanoKid can be changed by changing the ketal part of the NanoKid. Dr. James Tour used a variety of diols to make NanoProfessionals, such as NanoChef, NanoAthlete and NanoScholar. Furthermore, by hydrogenating the triple bond on the “waist” to a single bond, and coupling “hands” of NanoKids, the research team got NanoBalletDancers and NanoKid-Polymer respectively.

Electron cloud-based space-filling model of NanoProfessionals (Copyright: James M. Tour)
NanoCar
Other than NanoKids, the research group of Dr. Tour also built NanoCars by carbon-based molecules and won the first prize in the NanoCar Race in 2017.
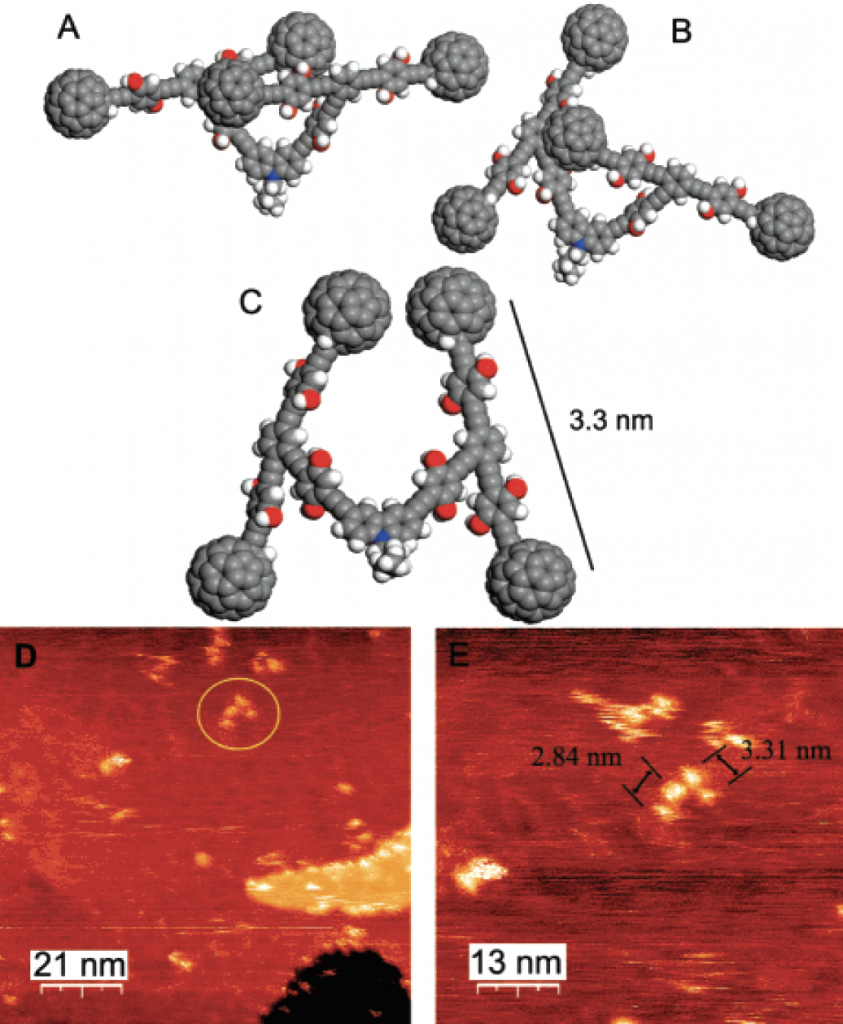
Three models of possible conformations of NanoCars under the scanning tunneling microscopy (STM) (Copyright: Organic Letters)
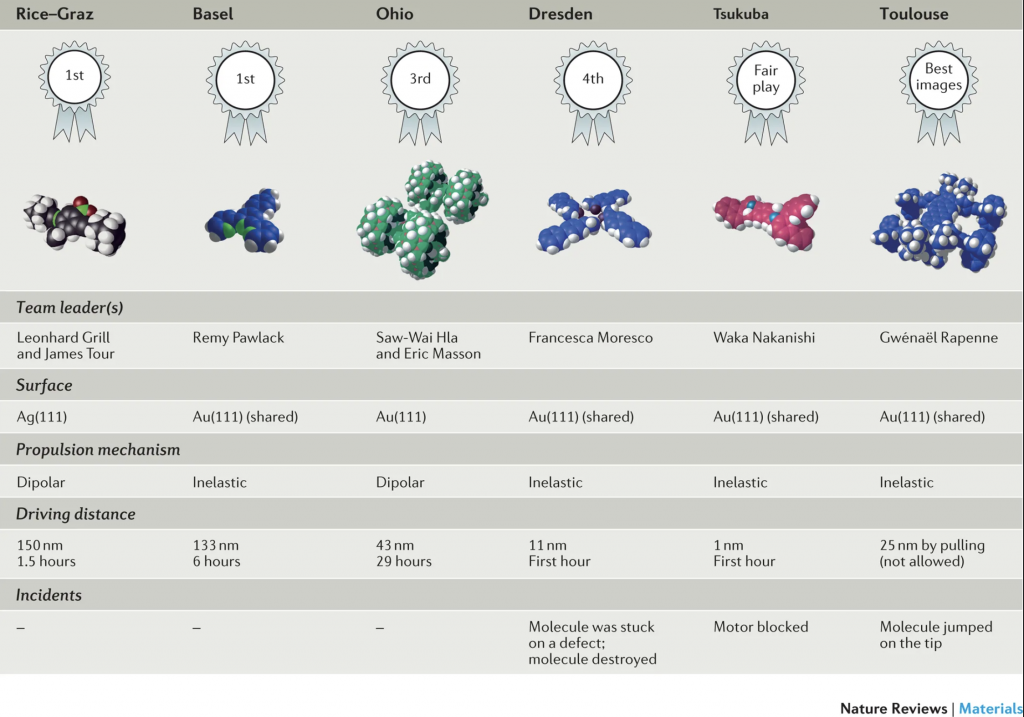
Summary of the NanoCar Race results. (Copyright: Nature)
Recently, many organic chemists use carbon atoms as building blocks to build molecules with unusual names. Such as “Broken Windowpane” which has a molecular formula of C8H12 and looks like a broken window, “Housane” which looks like a house and “Churchane” which looks like a church.
Is it a waste of taxpayers’ money?
Chemists have already synthesized the NanoKid, NanoCar and Broken Windowpane. In the future, chemists might build more interesting Nano-things. These research outcomes are very delighted, but some people might ask: Is it a waste of money? What is the meaning of these chemicals?
To synthesize a Broken Windowpane, chemists need to overcome an extraordinary intramolecular tension, to give birth to a NanoKid, researchers had to design and control the reaction accurately. “Beyond the molecular-sized domain, there is no conceivable entity upon which to tailor architectures that could have programmed cohesive interactions between the individual building blocks. It is at this size region that synthetic chemists have been inherently captivated; however, their fascination is rarely shared by the layperson.” Dr. Tour said. The Broken Windowpane might be adapted for more fantastic molecules, and the NanoCar might be used to deliver targeted drugs to a certain part of the body one day in the future. These molecules show that chemists can make whatever they want, and how magic chemistry is.

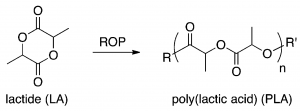 Figure 1. Chemical structures of lactide (monomer) and polylactic acid (polymer) . ROP stands for ring-opening polymerization (a type of polymerization). DOI:
Figure 1. Chemical structures of lactide (monomer) and polylactic acid (polymer) . ROP stands for ring-opening polymerization (a type of polymerization). DOI: