You may be aware of the role physicists and doctors play in diagnostic nuclear medicine, however you may not know that chemists also play a significant role in this area of science!
In 2019, Chris Orvig of the Medicinal Inorganic Chemistry Group at the University of British Columbia (UBC) created a new organic molecule for medical imaging. They also determined that their novel organic molecule has superior properties to similar molecules currently being used.
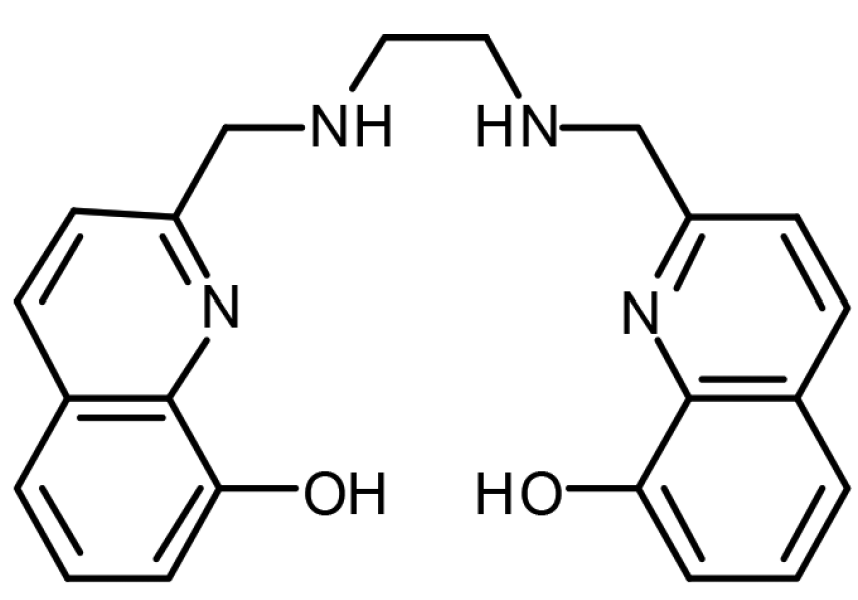
The molecule created by the inorganic chemistry group at UBC is simply known as H2hox, a hexadentate chelating ligand. What exactly does that mean? Let’s break it down piece-by-piece.
A ligand is a type of molecule that can bind onto a metal ion, like sodium (Na+) or calcium (Ca2+). In the case of H2hox, the metal ion it’s binding to is Gallium (Ga3+) because it is used in medical imaging.
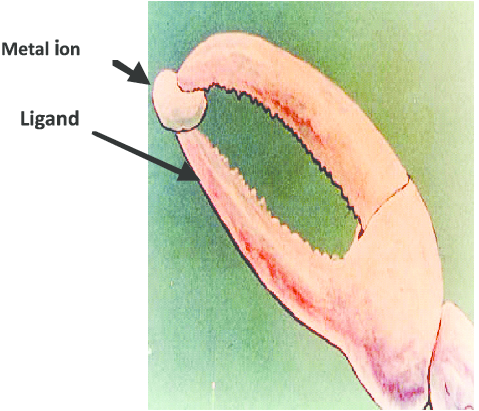
The word chelating comes from the latin root word chela, which means claw. This is because chelating ligands have multiple points of attachment to a metal ion, similar to a crab’s claw, making them significantly stronger binders to metal ions.
The word hexadentate comes from the latin root words hexa, which means six and dent, which means tooth. So a hexadentate chelating ligand has six attachment points, or teeth, that can grab onto a desired metal ion.
- A crab’s claw modelling the function of a chelating ligand.
- The pincer-like chemical structure of the chelating ligand H2hox..
Image sources (left to right): Research Gate, Orvig et al..
So why is H2hox used in medical imaging?
Molecules such as H2hox are used in a form of medical imaging known as Positron-emission tomography (PET). John Hopkins Medicine defines PET imaging as “using a scanning device (a machine with a large hole at its center) to detect photons (subatomic particles) emitted by a radionuclide in the organ or tissue being examined”.
PET imaging is primarily used to diagnose health issues related to biochemical processes occurring inside our cells, such as cancer. The radionuclide, or radioactive atom, of choice for H2hox is Gallium ions. Since ions alone cannot be used in imaging, due to their poor mobility through our cells and tissues, they are packaged together with small organic molecules, such as H2hox, before injection into human tissue.
So what makes H2hox better than the current available options?
H2hox is an advantageous ligand for Gallium PET medical imaging because…
- It can be easily synthesized (made) through only 1 reaction step.
- It has a strong affinity to Gallium, exhibiting significant radiolabeling (binding to Ga3+) in only 5 minutes with low amounts of ligand and under mild conditions (room temperature)
- The combined ligand and ion have excellent stability in vitro (inside cells) and in vivo (inside a beaker).
These combined properties make H2hox an effective and convenient molecule for Gallium PET imaging. Furthermore, Orvig’s research will act as a launching-off point for the development of even better ligands to improve the quality and ease of PET imaging and diagnosis.
I hope this news article educated you about medicinal inorganic chemistry through describing its role in medical imaging.
Literature cited:
Wang, X.; De Guadalupe Jaraquemada-Peláez, M.; Cao, Y.; Pan, J.; Lin, K. S.; Patrick, B. O.; Orvig, C. H2hox: Dual-Channel Oxine-Derived Acyclic Chelating Ligand for 68Ga Radiopharmaceuticals. Inorg. Chem. [Online] 2019, 58, 2275-2285. https://pubs.acs.org/doi/10.1021/acs.inorgchem.8b01208 (accessed March 22, 2020).
-Mark Rubinchik