If you are a coffee lover, you would probably know the naturally occurring substance, caffeine. But, were you aware that this substance is classified as a pseudoalkaloid?
Many pseudoalkaloids can often have biological activities like caffeine stimulates our nervous system. Ultimately, pseudoalkaloids can be used as building blocks to produce useful drugs.
In 2019, researchers at the University of British Columbia, led by Dr. Schafer, uncovered a new pathway to produce structurally simple terpenoid-alkaloids, which belong to pseudoalkaloids.
This study can be simply summarized as a reaction between a terpene and an amine with the help of a tantalum catalyst. But, let’s first explore key ingredients to deeply understand how the synthetic route works!
An organotantalum compound with a ureate salt
The researchers developed a catalytic reaction run by a metallic compound. Based on other known studies, they chose an organoctantalum compound to produce terpenoid-alkaloids. As like an engine is the heart of a car, the tantalum compound is an engine to drive reactions to the final products, terpenoid-alkaloids
The choice of a metallic compound is of course crucial. However, it is more important for the compound to have complete catalytic potential. How could a bare metallic compound become a complete catalyst? The answer is associating a metallic compound with a ligand such as organic molecules or salts, which can coordinate to a metal center. Of numerous possible candidates of ligands, the researchers found that a specific salt can improve the efficiency and selectivity of the bare organotantalum compound, thereby allowing it to have a complete catalytic ability.
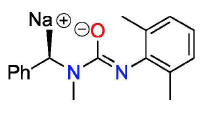
Figure 1.The ureate salt that improved selectivity and efficiency of the organotantalum compound, Ta(CH2SiMe3)3Cl2. Of several ureate salts, the above salt was the most suitable for this study due to its solubility.
Terpenes and anilines
As the name of final products, terpenoid-alkaloids, reflects the use of terpenes, one of key ingredients is a terpene, a naturally occurring molecule. By limiting the scope of terpenes to enantiopure limonene and pinene, the types of anilines were varied and reacted with the terpenes
Now, here comes a question. What is the consequence of mixing these ingredients together?
Fascinating results
This study is fascinating not only for the reason that a catalytic amination of terpenes is unexplored, but also the final products are not chaotic mixtures.
What does it mean by a chaotic mixture? Some catalysts have potential to alter an intrinsic structure of a staring substance. For example, if a catalyst was able to influence the chiral center of (R)-limonene by changing its stereochemistry, a reaction batch would contain both (S) and (R)-limonenes. Consequently, the occurrence of two products is equally probable.
Also, unexplored magical ability of the tantalum catalyst in the study allows anilines to react with one specific spot of an alkene moiety in terpenes. This astonishing selectivity gives a rise to one major product.
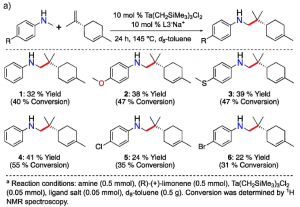
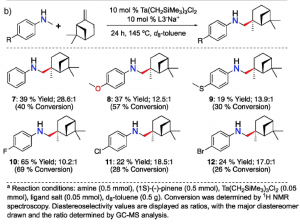 Figure 2.The reaction of an enantiopure limonene with six different anilines (left). The reaction of an enantiopure pinene with six different anilines (right). Both reactions result in high regio- and diastereoselectivity.
Figure 2.The reaction of an enantiopure limonene with six different anilines (left). The reaction of an enantiopure pinene with six different anilines (right). Both reactions result in high regio- and diastereoselectivity.
Reference
Dipucchio RC, Rosca SC, Athavan G, Schafer LL. Exploiting Natural Complexity: Synthetic Terpenoid‐Alkaloids by Regioselective and Diastereoselective Hydroaminoalkylation Catalysis. ChemCatChem. 2019;11(16):3871–6.
-Young Cho
