What if there’s more to caffeine than meets the eye? What if we can chemically change the way caffeine works to create a better version of itself.
If you are a coffee lover, you probably know the naturally occurring substance, caffeine, and its stimulating properties to our nervous system. Why and how does it happen?
Using a special type of reaction, the biological activity of caffeine can be transformed to suit a variety of different applications. Past studies can be improved by changing the materials needed and the methods used.
Dr. Schafer’s team, at the University of British Columbia, examines an approach to synthesize pseudoalkaloids (Fig. 1), chemicals present in caffeine, with great accuracy.
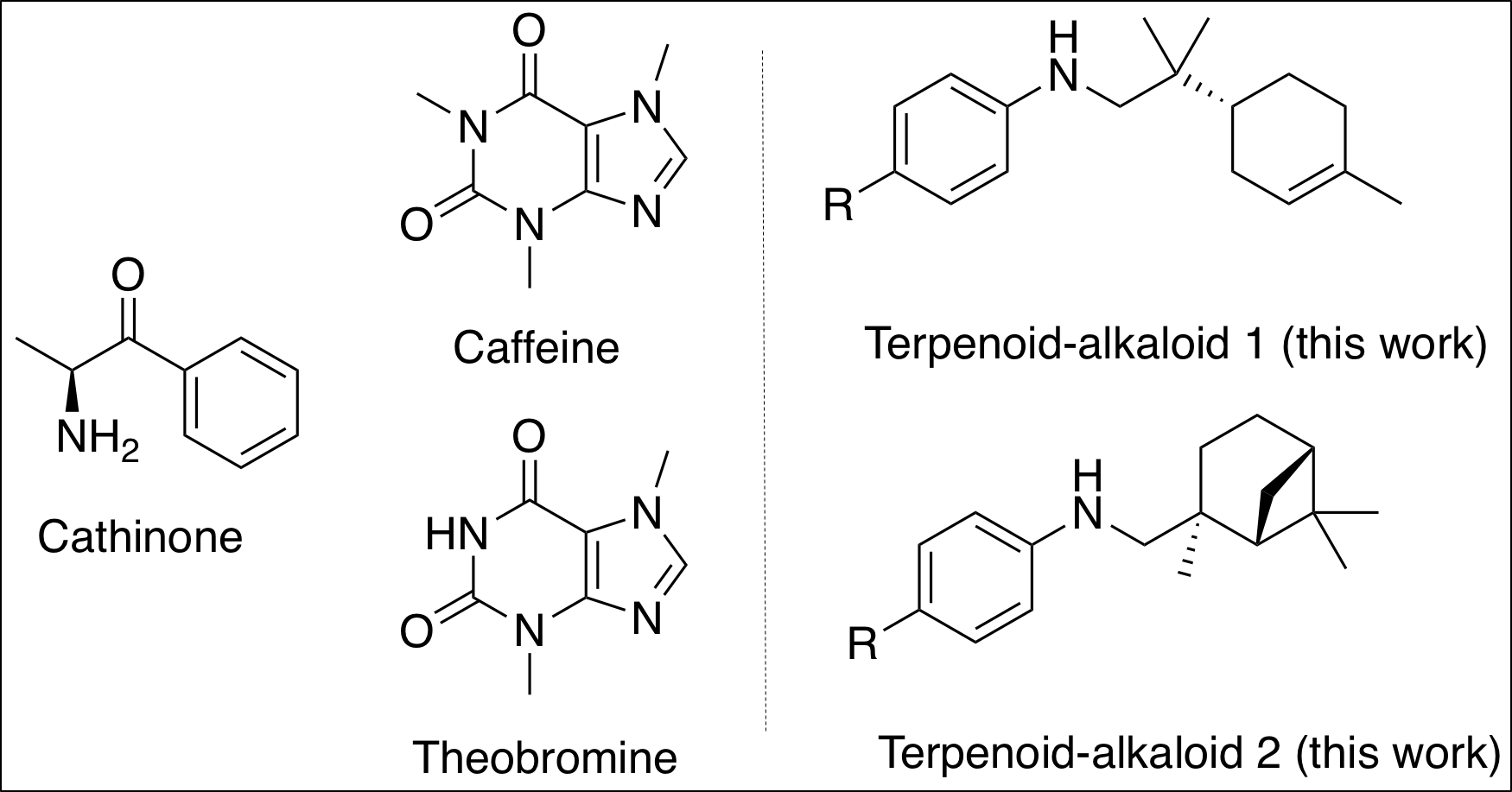 Figure 1. Examples of pseudoalkaloids. The pseudoalkaloids on the left are produced naturally or synthetically. The pseudoalkaloids on the right are the researchers’ target molecules. (molecules were drawn with ChemDraw 19.0; Credits: Wilson, Young, and Rachel)
Figure 1. Examples of pseudoalkaloids. The pseudoalkaloids on the left are produced naturally or synthetically. The pseudoalkaloids on the right are the researchers’ target molecules. (molecules were drawn with ChemDraw 19.0; Credits: Wilson, Young, and Rachel)
Pseudoalkaloids can be artificially made, but time-consuming multiple synthetic steps limit the production of pseudoalkaloids. Importantly, the challenge lies in the reactivity of the starting materials, and whether it can react to produce the desired pseudoalkaloid without byproducts.
Similar studies show different products have formed, which proves to be a problem.
Existing studies experimented with different types of metal catalysts showing potential improvement for the results. A tantalum catalyst, a tool that speeds up the reaction, is tested on a reaction to observe its effectiveness on producing the final product.
An idea was proposed to add molecules that bind to the tantalum catalyst. This binding molecule improves the reactivity of the catalyst, thereby converting as many initial materials as possible to the final product.
Of many possible starting materials, terpenes (Fig. 2) were used for the synthesis of pseudoalkaloids. In addition to the inactive alkene groups in terpenes, various chemical structures make them attractive starting substrates to explore a new synthetic route.
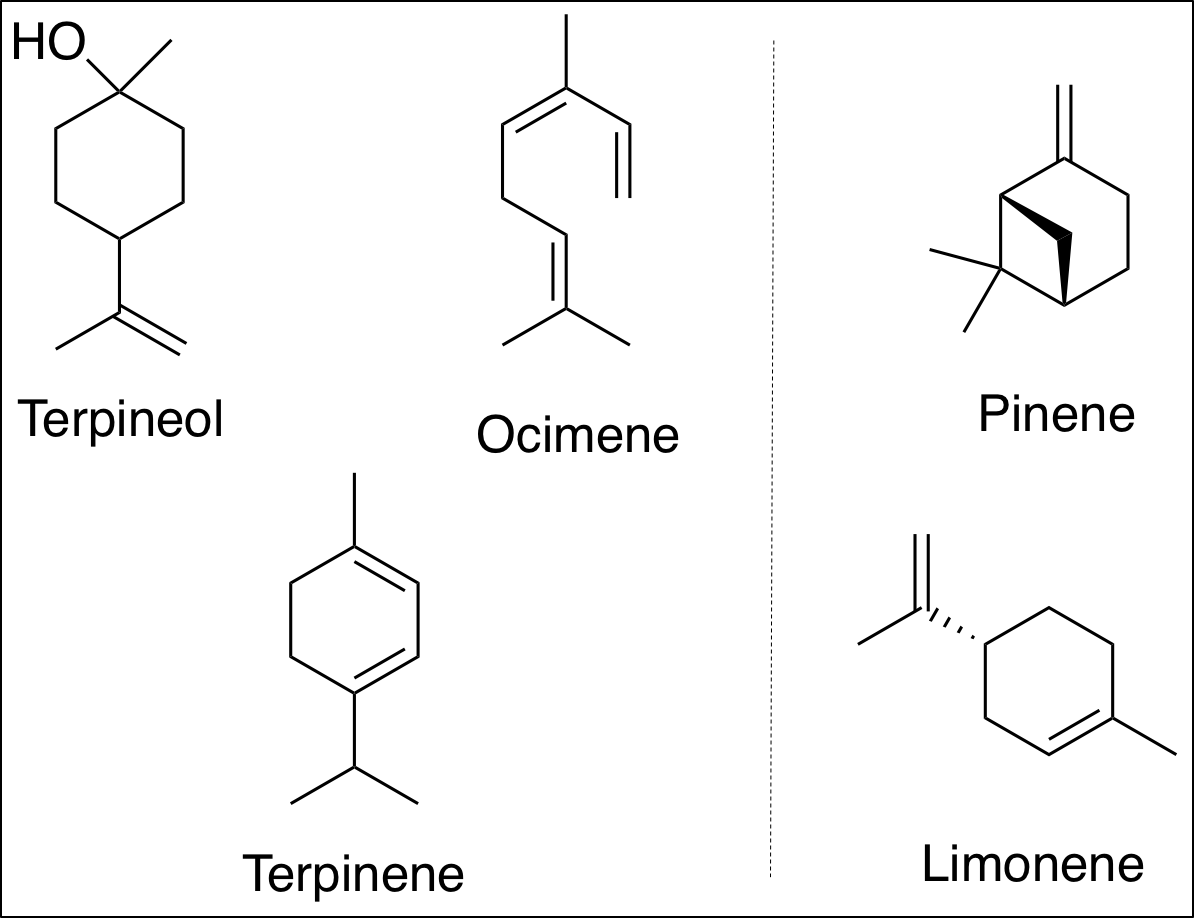 Figure 2. Examples of terpenes. Terpenes are naturally occurring organic molecules, produced by plants. The terpenes on the right are starting materials chosen by the researchers. Every terpene has unsaturated functional groups which may react with other molecules. (molecules were drawn with ChemDraw 19.0; Credits: Wilson, Young, and Rachel)
Figure 2. Examples of terpenes. Terpenes are naturally occurring organic molecules, produced by plants. The terpenes on the right are starting materials chosen by the researchers. Every terpene has unsaturated functional groups which may react with other molecules. (molecules were drawn with ChemDraw 19.0; Credits: Wilson, Young, and Rachel)
Another key building block, an amine, can be any, as long as the nitrogen atom of an amine is directly bound to one hydrogen atom. However, a clever choice can even make the final products useful building blocks, allowing further modifications (Fig. 3)
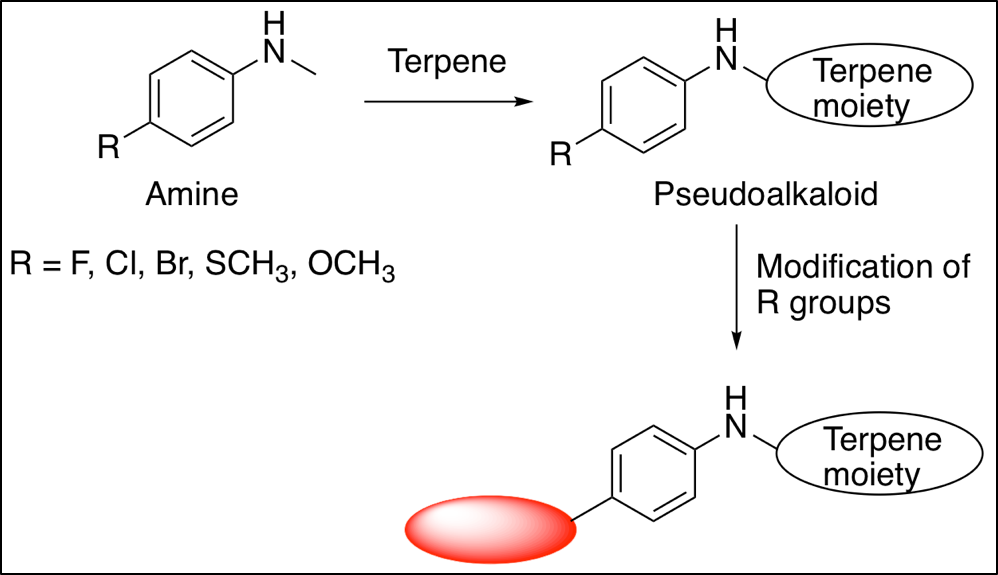 Figure 3. Amines with varying R groups. After reacting with a terpene, the R group of pseudoalkaloid can be further modified to form a new molecule. (molecules were drawn with ChemDraw 19.0; Credits: Wilson, Young, and Rachel)
Figure 3. Amines with varying R groups. After reacting with a terpene, the R group of pseudoalkaloid can be further modified to form a new molecule. (molecules were drawn with ChemDraw 19.0; Credits: Wilson, Young, and Rachel)
The catalytic reaction between amines and terpenes with the tantalum catalyst showed great selectivity. Without the help of the tantalum catalyst, an amine could potentially select any active spot on a terpene and react with it, causing a mixture of pseudoalkaloids at the end of the reaction.
However, the tantalum catalyst results in one dominating product. Although the final pot contains some residual starting materials, the target pseudoalkaloids are the major product that can be easily isolated.
By constructing a pathway to ultimately arrive at the designated point, new and better options can be achieved. Caffeine is one of many that can be innovated upon.
Reference
Dipucchio RC, Rosca SC, Athavan G, Schafer LL. Exploiting Natural Complexity: Synthetic Terpenoid‐Alkaloids by Regioselective and Diastereoselective Hydroaminoalkylation Catalysis. ChemCatChem. 2019;11(16):3871–6.
-Group 9 (Wilson, Young, Rachel)
