Cancer is complicated, there’s no doubt about that. From its onset to its invasive prevalence, preventing the progression of each stage seems like a never-ending battle. Be that as it may, with the advent of new chemical entities, this fight has become a lot easier.
In 2019, the Orvig group in the University of British Columbia were able to design and create a new molecule with a variety of applications. One of which is in cancer imaging, which is what makes their compound particularly astonishing. Further, the authors have reported that their chemical species is stable in, and is removed quite easily from the body. Though to understand the importance of their findings, it is vital to start at the very beginning.

Dr. Orvig (Top row, left) and his medicinal inorganic chemistry group
Within the realm of diagnostic medicine, imaging is a vital facet. The goal of imaging is to ultimately produce a viable image of the body. Positron emission tomography (PET) is an imaging method used to generate images of the chemical changes that happen in tissue. These scans often rely on the properties of the radiation emitted by a radioactive isotope. In this instance, the radioisotope used is Gallium-68 (68Ga), which is a readily available and versatile tracer species. Though, to harness the tracer capabilities of 68Ga, it would have to exist in the form of a compound. This is where the Orvig group plays an important role.
They were able to synthesize a ligand with the ability to interact with the 68Ga. A ligand is essentially a molecule that can bind to a metal center, forming a metal complex. This leads to the generation of a stable species that can exist in solution and thus can move around the body. What the group was able to show was that this novel ligand had a high affinity for 68Ga even at low concentrations. Their ligand is an acyclic hexadentate ligand, named H2hox. Though the name is inherently complicated, it does make sense once it is explained in some depth.
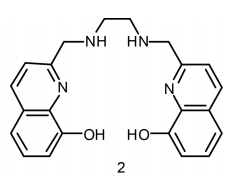
The Orvig group’s hexadentate chelating ligand
A hexadentate ligand is a ligand that coordinates to the metal center at 6 different positions. What this means is that there are 6 different points on the one molecule that bond with the metal center. Furthermore, the ligand is said to chelate the metal center. This term is generally given to a ligand that binds to a central metal atom at two or more points. Fortunately, this ligand is advantageous in a number of different ways.
The authors have stated that this compound is easy to synthesize, removing a number of potentially challenging synthetic strategies typically associated with 68Ga chelating species. Their initial starting materials were also inexpensive and are actually available online. To put this into perspective relative to some of the other ligand synthesis methods, it’s like baking a box cake versus baking a cake from scratch. The former is simple and quite easy to do, while the latter is a lot harder and is a lot more intensive. Ease of synthesis is an important feature, as it can affect the commercial applicability of the chelating ligand. Fortunately, their synthesis strategy is also mild.
What was also found is that their ligand is more stable than other 68Ga chelating ligand species. One advantage reported is with respect to acids and bases, where it was found that the complex is actually stable in both. It was found that there exists a single species, [68Ga(H2hox)]+, within a pH range of 1-11. Moreover, the group assessed the conditional stability of the complex. Again, these findings reinforce the advantages of their chelating ligand, H2hox.
Stability studies conducted in the sternum of mice and dynamic imaging PET studies also suggest that their compound is stable in the body and in a vial. This impressive aspect correlates with the enhanced stability of the H2hox metal complex. It was also removed from the mouse relatively quickly.
As a result of their success, their chelating species surpasses any ligand currently used as a 68Ga chelator. Not only have they managed to add to the current library of chelators, but they have also developed a convenient toolkit radiopharmaceutical compound.
Reference:
Wang, X.; De Guadalupe Jaraquemada-Peláez, M.; Cao, Y.; Pan, J.; Lin, K. S.; Patrick, B. O.; Orvig, C. H2hox: Dual-Channel Oxine-Derived Acyclic Chelating Ligand for 68Ga Radiopharmaceuticals. Inorg. Chem. [Online] 2019, 58, 2275-2285. (Accessed: March 23, 2020).
https://pubs.acs.org/doi/abs/10.1021/acs.inorgchem.8b01208
– Akash Panjabi