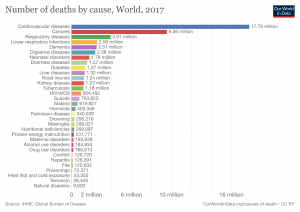
Cancer is a disease that killed 8.9 million people by 2016, and it is very hard to detect. For someone suffering cancer, they may want to detect it as early as possible so they can be treated. In the past, doctors have to take a patient’s tissue. With naked eyes, the doctor has to search for tiny cancer monsters in this tissue under a microscope, which is like searching a needle in an ocean. Even for highly-experienced doctors, this process is time-consuming and has a high chance of miss-detecting.
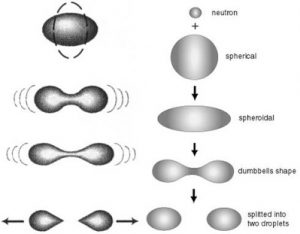
Now there are radioactive chemicals that can efficiently detect cancer cells. It works like this. There is a blue-colored chew-chew train in these chemicals called a positron, which collides with the red chew-chew train in your body called an electron. When they collide, they annihilate each other and make huge lightning like in a nuclear bomb. These lights are so strong that they can penetrate through your body and be captured by our detecting machine. So you might wonder, how do we know if there are cancer monsters then? Well, the cancer monsters can eat away light, so when the detecting machine receive light from areas of the patient’s body where cancer cell is present, the picture coming out would be much, much darker.
But here is another problem–––the radioactive chemicals are so dangerous that they can destroy your body, which is also why we make nuclear bombs out of them. Therefore scientists have found types of these chemicals that only explode for a few hours, on top of them is something called Germanium, it is not made in Germany, but you could memorize it that way. It’s not just normal Germanium, but a thin, thin Germanium that is a little lighter than the normal Germanium.
But the problem has not been solved. What if the Germanium jumps around your body and destroys everything? We need a claw that clamps onto this Germanium to transfer it into the patient’s body and hold it in the spot. There used to be many candidates, but they can only function at small ranges of pH outside your body. Now you must be confused; what exactly is pH? Well, think of a pool of sticky bubble gums. These gums are called H. When the pH is low, there are a lot of gums in the pool, so when the claw enters the pool, the gum will stick onto the claw, now the claw surface becomes soft, and the Germanium just slips away. Unfortunately, the human body is such a low pH pool. No claw has been able to hold the playful Germanium under every condition. Surprisingly, Dr. Ovrig just made this new big claw in his lab that would hold onto the Germanium. When they tested it in different pools, even one similar to the human body, the Germanium stayed happily inside the claw. If you are curious about the name of this claw, it’s called Ga(hox).
The researchers then brought this claw into practice. They used the claw and germanium combination on a mouse, and it easily detected cancer monsters in the heart within a short 1 minute. The monsters in the liver and bladder are also exposed after 1 hour. Now, patients no longer have to worry about the long period of cancer examination. Besides, this technique can detect even tiny amounts of cancer monsters. Therefore, even in the very early stage of cancer, the claw-germanium combo can detect cancer and allow treatment before the cancer monster goes rampart.
Reference
Wang, X.; De Guadalupe Jaraquemada-Peláez, M.; Cao, Y.; Pan, J.; Lin, K. S.; Patrick, B. O.; Orvig, C. H2hox: Dual-Channel Oxine-Derived Acyclic Chelating Ligand for 68Ga Radiopharmaceuticals. Inorg. Chem. [Online] 2019, 58, 2275-2285. (Accessed: March 23, 2020).