Recently, the outbreak of new coronavirus has seriously affected people’s lives in countries and even around the world. New coronaviruses can be transmitted through air and contact. Symptoms include fever, cough, and difficulty breathing. In order to completely solve this infectious disease, it is very important to find the primary case.
Source:https://www.heywood.org/education/covid19-coronavirus-updates
Initially, Chinese scientists believed that the source of the virus was the South China Seafood Market in Wuhan, China. When people eat wild animals that contain the virus, the non-pathogenic version of the virus jumps from animal hosts to humans and then evolves into the current pathogenic state in the population. For example, the RBD structure of some coronaviruses from pangolins, armadillo mammals found in Asia and Africa, is very similar to SARS-CoV-2. Coronavirus from pangolin may have been transmitted to humans either directly or through an intermediary host such as a civet or ferret.

Source:http://m.cyol.com/content/2020-03/05/content_18413469.htm
Most people in this epidemic believed that this new coronavirus should be derived from flying mammals such as bats. Many people also criticized them, and even many people targeted the bats directly, thinking that those who eat bats Caused the disease. However, this is actually questionable. Although it is possible that bats can directly infect humans, so far, most of the time, bats are not the direct source of infection but are adapted to spread to humans through the intermediate source of infection. This can be seen from the case report published in the top medical journal “The Lancet”.

Source:https://news.sky.com/story/how-did-coronavirus-start-scientists-tackle-the-conspiracy-theories-11959500
Researchers from institutions such as the Xishuangbanna Tropical Botanical Garden of the Chinese Academy of Sciences recently published a paper in the form of a preprint, saying that they analyzed the genomic data of 93 new coronavirus samples in 12 countries on four continents and found that they contained 58 haplotypes, which are related to the South China Seafood Market The associated patient sample haplotypes were H1 or its derivative types, while the more “older” haplotypes such as H3, H13, and H38 came from outside the South China seafood market, confirming that the new crown virus of the South China seafood market was transmitted from elsewhere In perspective.
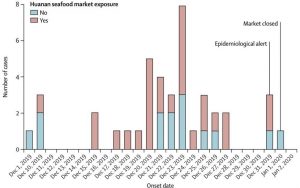
Source:https://meaww.com/coronavirus-did-chinese-officials-downplay-extent-of-deadly-wuhan-outbreak-in-early-stages
Finding an “index case” is equivalent to finding a weapon that can cut the epidemic from the source. But in the long history of humans fighting the epidemic, few “index cases” have been found. On the one hand, the timeline of the index case and first case are not necessarily the same, which makes the process of tracing like a needle in a haystack. In addition, the chain of evidence tracking “index case” is difficult to finalize and will always be repeatedly overturned and readjusted.
To this day, the epidemic of AIDS, Ebola, SARS and so on has never clearly identified the “index case” in the strict sense. From the perspective of the development of the global epidemic, it is still of great significance to curb the development of the epidemic while researching and developing effective drugs and vaccines and controlling the development of the epidemic in a timely manner. However, the new Coronavirus may be the same as AIDS and SARS. There is no way to accurately find the first human it infected.
Yicheng Zhu