
Four elements newly found in 2016 source:International Union of Pure and Applied Chemistry
Have you ever curious about the abundance of elements in this world?
Research groups in Japan, Russia, and the USA published their discovery of elements 113,115,117 and 118. On November the 28th of 2016, International Union of Pure and Applied Chemistry (IUPAC) has formally approved the name of these elements as Nihonium (Nh), Moscovium (Mc), Tennessine (Ts), and Oganesson (Og). These four elements completed the 7th row of the periodic table and acted as an important stepping stone toward “superstable-elements” which are going to be influential in the future studies.
Element 113, Nihonium (Nh), which called “The first element found in Asia” was found by Riken Center of Accelerator-Based Science in Japan. Joint Institute of Nuclear Research discovered three other elements of Moscovium (Mc), Tennessine (Ts), and Oganesson (Og) credited to Russia and the United States. After five months of public review, IUPAC eventually added them to the 7th row of the periodic table.
These four elements were classified as “super-heavy” elements with more than 104 protons. They were synthesized by using particle accelerators to fuse one nucleus to the other. Further experiments proved the existence of these elements by reproducing the synthesis procedures. However, the life of these “man-made” elements seem to be too short for further discovery. “A particular difficulty in establishing these new elements is that they decay into unknown isotopes very fast.” Said Paul Karol, chair of the IUPAC’s joint working party. Nihonium has a half-life of 20 seconds, which was the longest among the newly found elements. Moscovium and Tennessine have an even shorter half-life, which is only 220 milliseconds and 78 milliseconds respectively.
Example of Super-heavy element. source:Vanderbilt University
What is the purpose of discovering these elements since they disappear almost right after they are produced?
There are “islands of stabilities” which describe certain super-heavy elements that are very stable when they have a certain number of protons or electrons, even though they are huge. Scientists believe that the next island will be in the 8th row of the periodic table. “the alleged but highly probable ‘island of stability’ at or near element 120 or perhaps 126.” Said by Paul Karol. These “Island of Stabilities” can stay from minutes to years which will be meaningful to study their chemistry.
Although the life of these newly found elements is way too short to have a practical use, they are the sign of getting closer to the “Island of Stability” of “super-stable” heavy elements. Those “super-stable” radioactive elements are worthy of studying and could have a lot of industrial applications. For example, they might be useful as a stockpile of nuclear energy to maintain national safety. The discovery of these elements gave hope to scientists and encourage them to further discover the ultimate limit of the periodic table. Hopefully, they will be able to discover some stable super-heavy elements that are influential and have significant practical uses soon. The study of new elements would eventually be the breakthrough point of modern chemistry!
–Vicky Gu






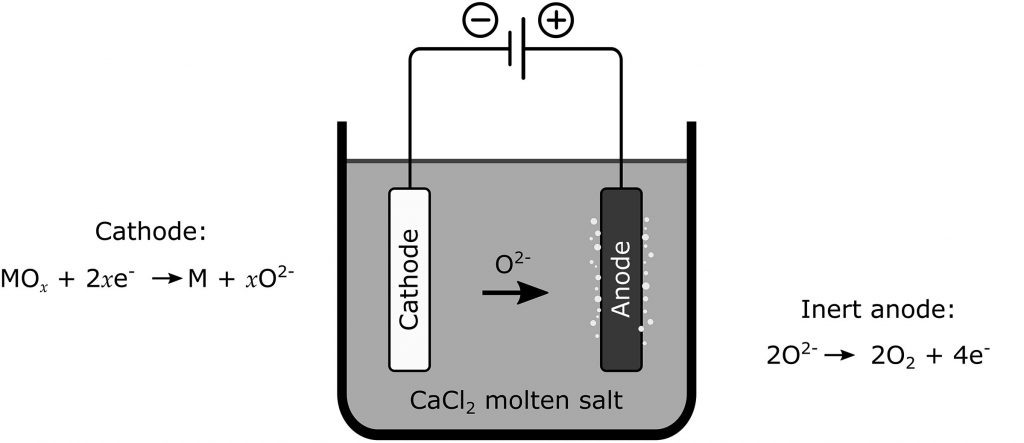
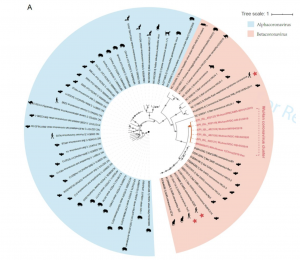 Phylogenetic tree
Phylogenetic tree 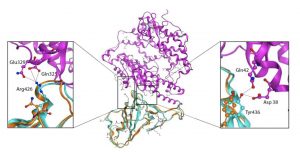 Cα RMSD of 1.45 Å on the RBD domain compared to the SARS-CoV S-protein structure
Cα RMSD of 1.45 Å on the RBD domain compared to the SARS-CoV S-protein structure  Source:
Source: 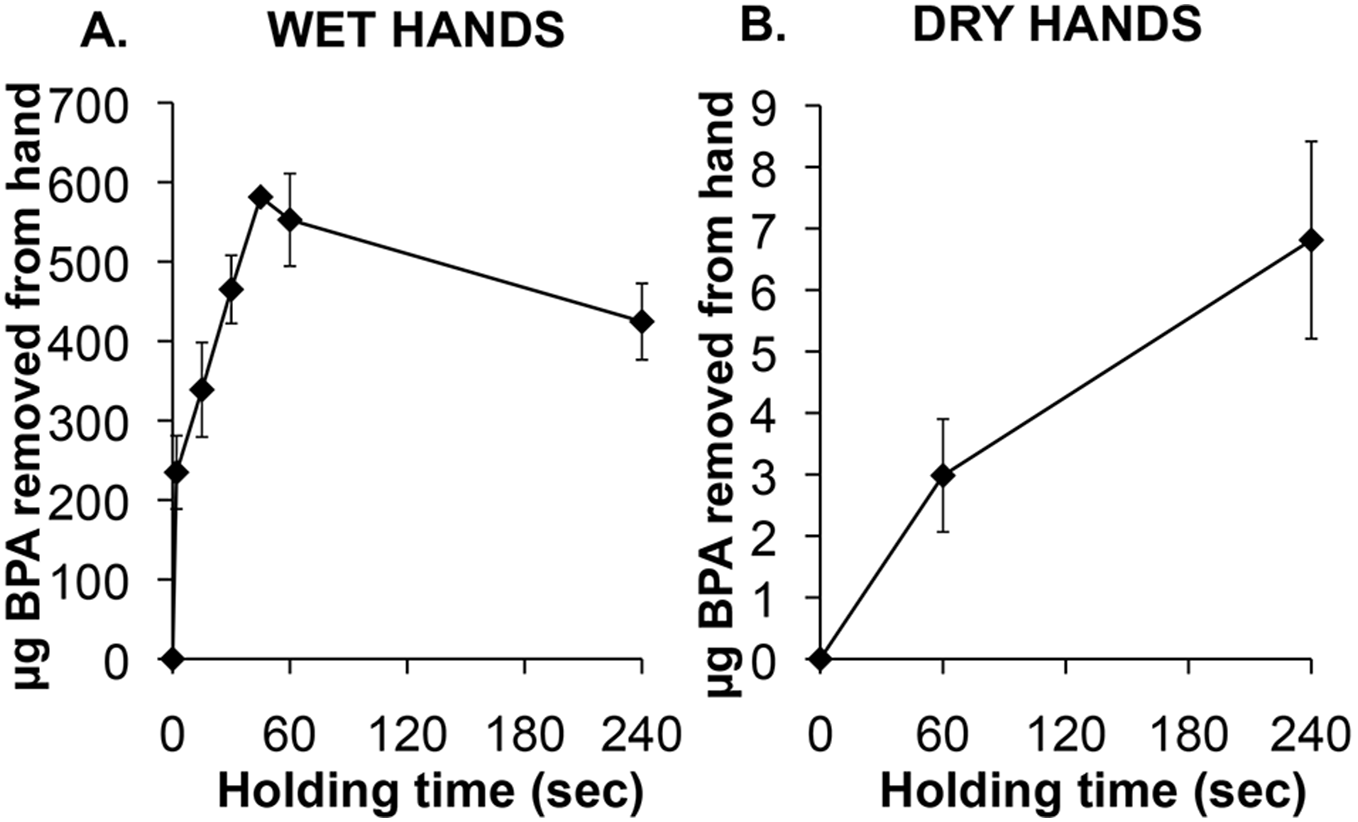 Source:
Source: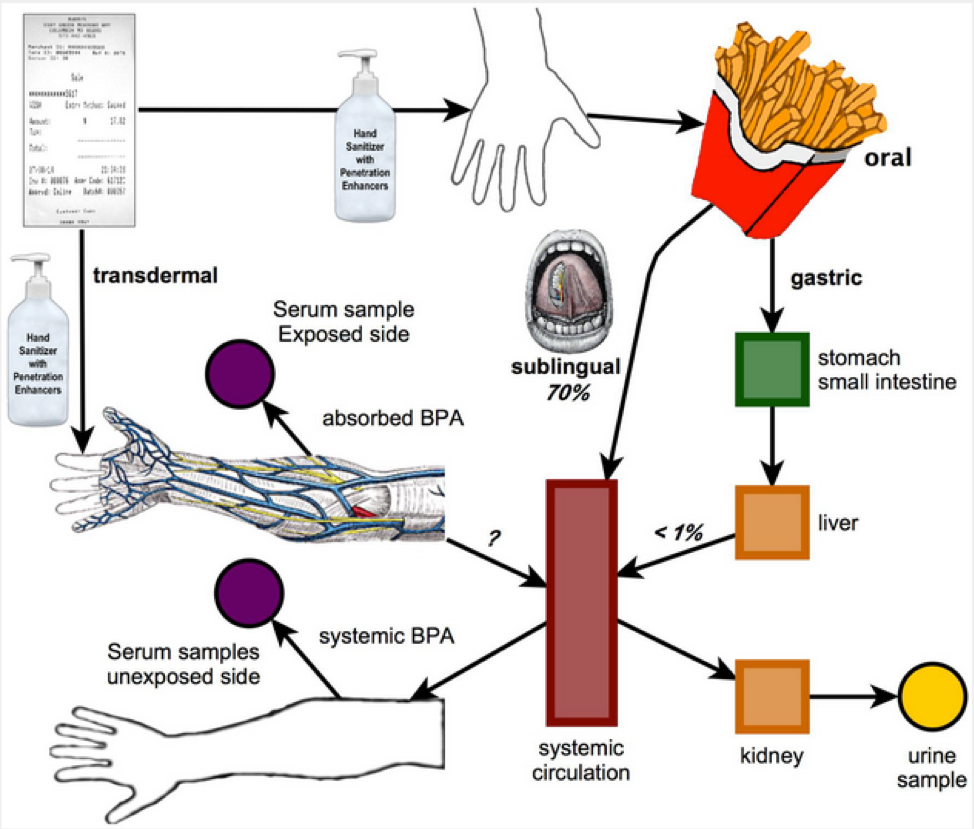 Source:
Source: