What do the effects of the chemicals in caffeine tell us? Researchers at the University of British Columbia, led by Dr. Laurel Schafer, answers this question, in 2019, by developing an efficient method to produce a similar chemical, known to be used in various applications.
A starting material reacts with a chemical in a determined set of conditions to yield a single product. This product identifies itself as a class of pseudoalkaloids.
Pseudoalkaloids can be described by their use for biological activity and exhibit enhanced properties compared to alkaloids, such as cancer treatment. Pharmaceutical industries often investigate biological activities of alkaloids to use them for drugs.
If you are a coffee lover, you probably know the naturally occurring substance, caffeine, and its stimulating properties to our nervous system. Caffeine is classified as a pseudoalkaloid.
Using a special type of reaction, these alkaloids can be created with materials that are compatible with each other. Past studies can be improved to target specific types of pseudoalkaloids by changing the materials and methods used.
Pseudoalkaloids can be artificially made, but time-consuming multiple synthetic steps limit the production of pseudoalkaloids. Importantly, the challenge lies in the reactivity of the starting materials, and whether it can react to produce the desired pseudoalkaloid without byproducts.
This proves to be a challenge because structurally complex or large chemicals have a hard time to mingle with their pairs. As such, this gives rise to multiple unwanted products, as seen in similar studies.
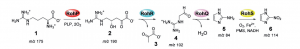
The solution lies within the tantalum catalyst, a tool that speeds up and controls the reaction, which is tested on a reaction to observe its effectiveness on producing the final product. Existing studies experimented with different types of metal catalysts and show a high potential for great results.
An idea was proposed to add molecules that bind to the tantalum catalyst. This binding molecule improves the reactivity of the catalyst and proceeds the reaction, thereby converting as much initial material as possible to the final product.
After many attempts of finding the perfect combination of chemicals to react in the optimal conditions, Dr. Laurel Schafer’s group has synthesized the desired pseudoalkaloid of interest. For public use, the product is isolated and purified easily, since the reactivity of the reaction is maximized.
The experiment is deemed successful as it tackled all the problems faced from past researchers. One example is the selectivity of the reaction, where the reaction conditions can structurally change the final product and display undesired applications.
Evidence proves the benefits of developing pseudoalkaloids, like caffeine, and hold significant demand in the public. It is possible to design synthetic methods to produce different pseudoalkaloids with caffeine in mind.
Reference
Dipucchio RC, Rosca SC, Athavan G, Schafer LL. Exploiting Natural Complexity: Synthetic Terpenoid‐Alkaloids by Regioselective and Diastereoselective Hydroaminoalkylation Catalysis. ChemCatChem. 2019;11(16):3871–6.
-Group 9 (Wilson, Young, Rachel)


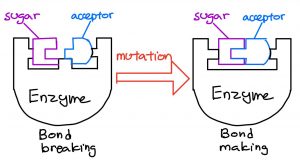
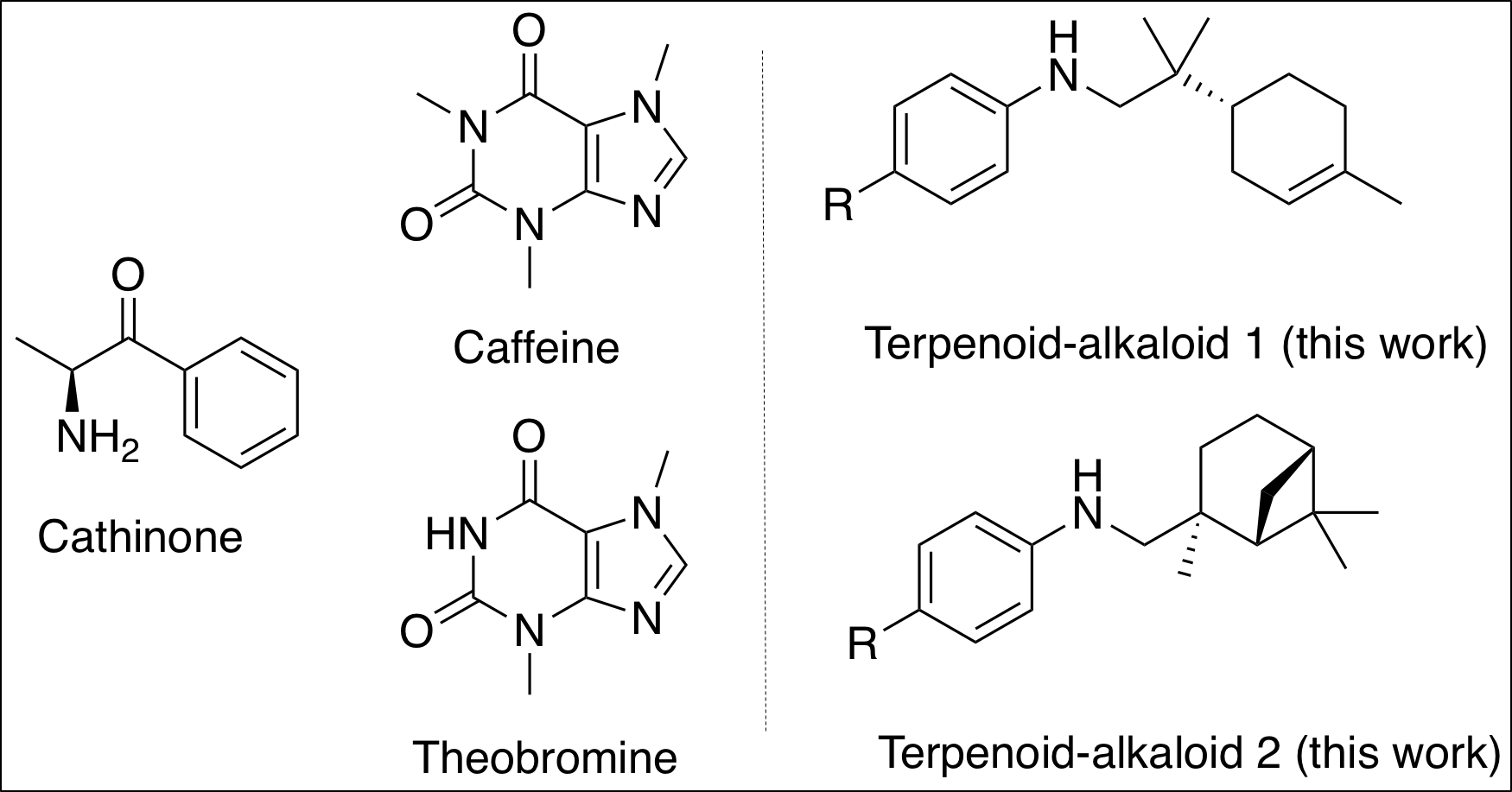 Figure 1.
Figure 1.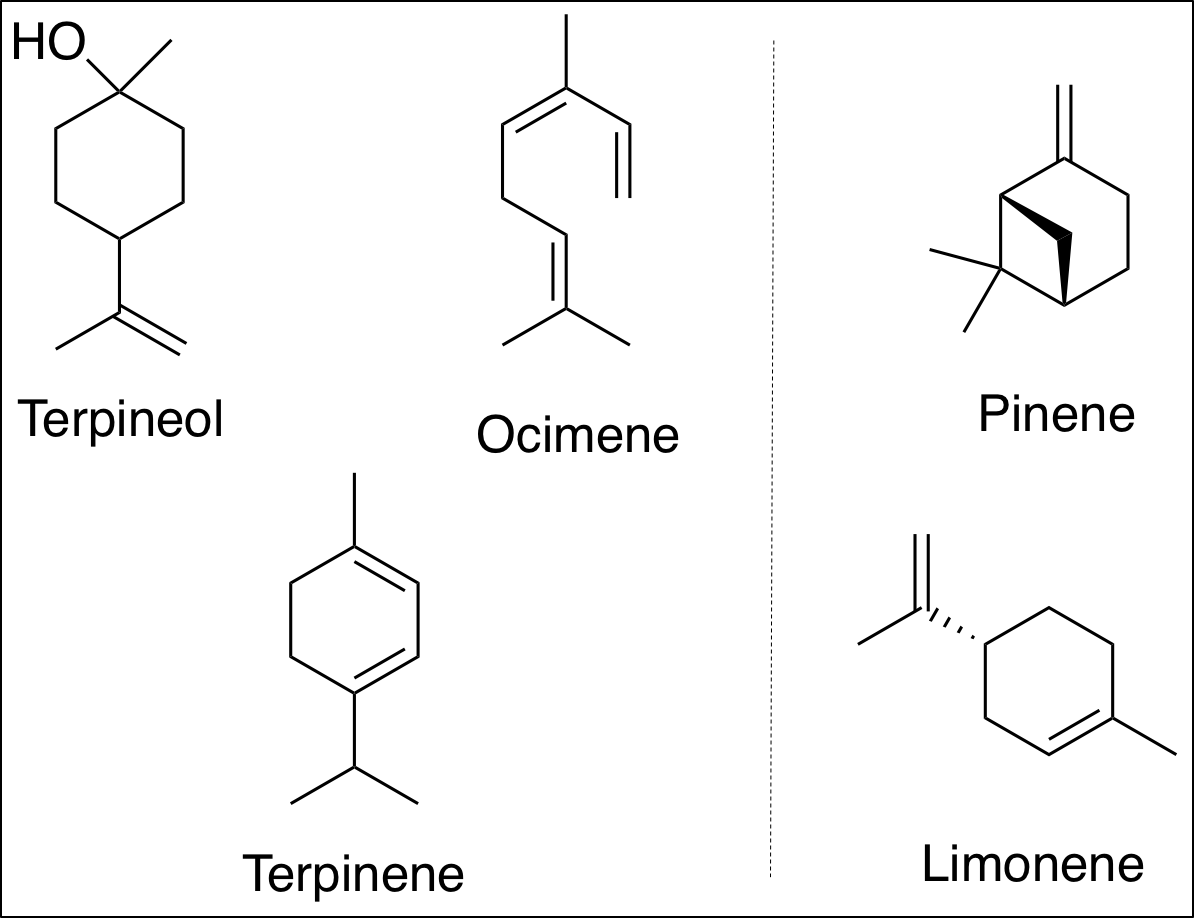 Figure 2.
Figure 2. 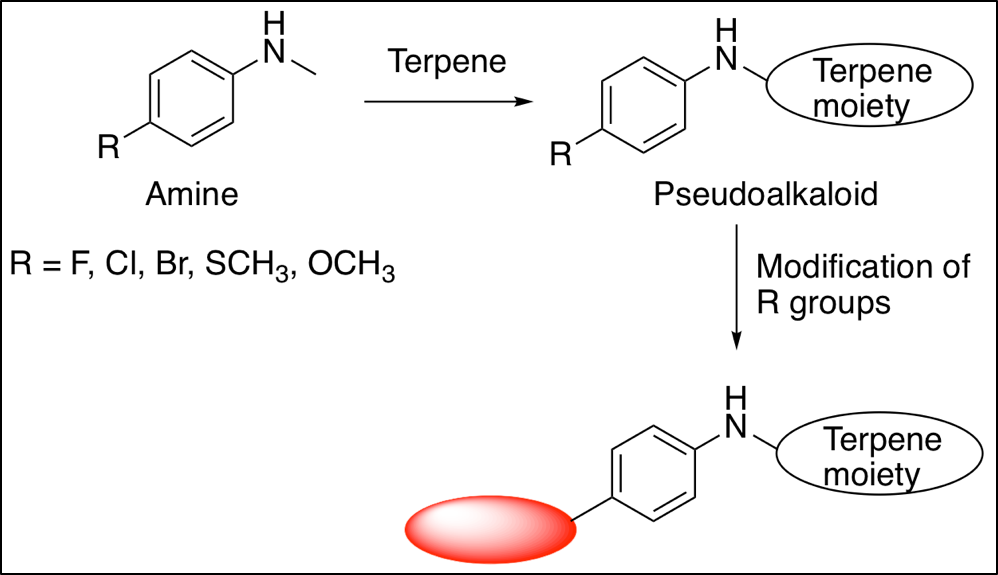 Figure 3.
Figure 3. 
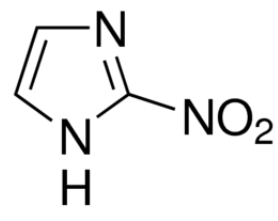
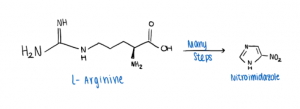



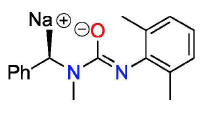
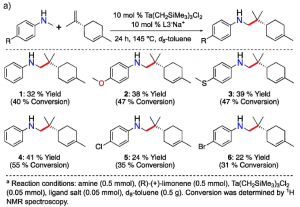
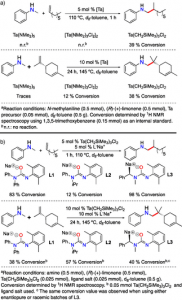
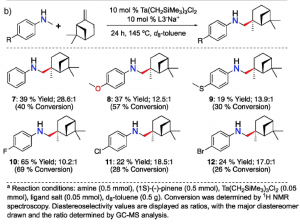 Figure 2.The reaction of an enantiopure limonene with six different anilines (left). The reaction of an enantiopure pinene with six different anilines (right). Both reactions result in high regio- and diastereoselectivity.
Figure 2.The reaction of an enantiopure limonene with six different anilines (left). The reaction of an enantiopure pinene with six different anilines (right). Both reactions result in high regio- and diastereoselectivity.