Since the AlphaGo defeated the world Go champion in 2016, artificial intelligence (AI) has revolutionized many fields in our life. It has also become a new star in science and opened up new possibilities to solve the most complicated problems by computers.
Not long ago, a group of chemists and computer scientists built the “AlphaGo” in chemistry. Published in Nature, they designed an AI tool that can plan organic retrosynthesis faster than any similar programs. In a double-blind AB test, chemists on average considered the AI-generated routes to be equivalent to reported literature routes. This achievement may shorten the process for drug designs and accelerate pharmaceutical research in the future.
Retrosynthetic analysis is the canonical technique used to plan the synthesis of organic molecules. In the past, scientists have also tried to design retrosynthetic routes by computers. Although this method can improve the synthesis efficiency, the traditional algorisms are slow and have many errors.
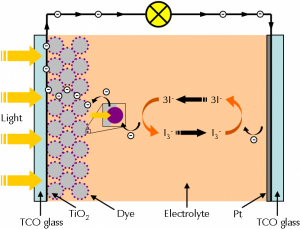
However, the AI developed by Segler’s group speeds up this process significantly. Described by Segler, the synthesis of molecules is very similar to playing Go: Each molecule can be constructed by synthons which are the “playing pieces”. Computers then design routes for the synthons and combine them together.
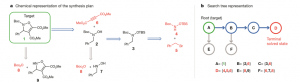
Figure 1. Translation of the traditional chemists’ retrosynthetic route representation to the search tree representation. Source: Nature
In the research, the AI tool learned more than 12 million single-step chemical reactions by the deep neural network. This can help AI predict any chemical reactions in the synthetic sequence. AI can also apply the neutral network repeatedly to plan routes and construct synthons until ending up with accessible starting reagents.
So far, much research focuses on combing deep neural networks with Monte Carlo tree search (MCTS). Monte Carlo tree search is a method widely used in video games to evaluate the movement of an object. After the player moving one step in the game, the computer will simulate infinite possibilities that may occur and choose the best step. Similarly, computers can also use this network to find the optimal method in organic synthesis.
In a trial test, Waller’s group used this algorism to propose a six-step synthesis for a precursor used in Alzheimer’s treatment. It turns out the AI designed the same route as the literature in less than 5.4 seconds.
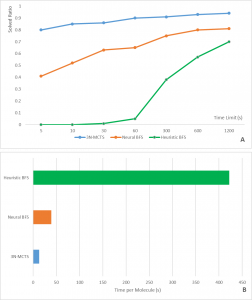
Figure 2. Comparison between the MCTS and two traditional algorisms: Neural and Heuristic Best First Search (BFS). (A) Performance characteristics of the different search algorithms by finding synthesis routes. (B) Amount of time per molecule to find the optimal route. Adapted from: Nature
More surprisingly, the AI can perform as good as organic chemists in predicting synthetic routes for novel drugs. Segler and his team invited 45 world-leading organic chemists from Germany and China to examine two potential synthetic routes for nine molecules. One route was designed by AI and the other by humans. Results show that chemists cannot distinguish between the two methods.
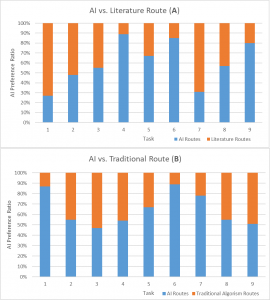
Figure 3. Double-blind AB testing of AI route (MCTS) against literature and traditional (BFS) routes. AI route is as preferable as literature routes and much better than traditional methods. (Original)
“What we have seen here is that this kind of artificial intelligence can capture this expert knowledge,” says Pablo Carbonell, a famous computational chemist at the University of Manchester. He describes the effort as “a landmark paper”. Maybe in the near future, AI will make a revolutionary change in chemical research and industry.