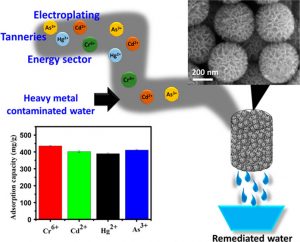
Carbon nanostructures have the potential to be used as a new form of water purification. A team of researches out of the Indian Institute of Technology Bombay have shown the potential to clean out Mercury, Cadmium, and Chromium ions safely from drinking water.
Source: Moronshing et al.
The study, which came out at the end of December 2019, shows that three-dimensional nanostructured carbon florets (NCFs) are tightly packed enough to inhibit heavy metal ions such as Hg2+ and Cd2+ form passing through, while allowing the much smaller H2O molecules to pass through easily. This material is particularly good at adsorbing out multiple heavy metal ions simultaneously, making it uniquely qualified for practical use in water filtration systems.

Source: Moronshing et al. (adapted)
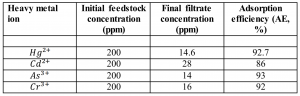
As you can see in the above image, the NCFs plug the narrow pathway for the contaminated water to reach the receiving flask. As the water molecules pass through, the pollutants are almost entirely blocked. The chart bellow specifies that as much as 93% of the heavy metals ion are removed from the solution, simply by passing through this NCF filter.
Source: Moronshing et al. (adapted)
Ease of Implementation
The most interesting aspect of this discovery is how easy it is to use. No energy is required to enable these filters, the water source simply passes through, and between 80% to 90% of the ions are instantly trapped. The study further shows that NCFs are easy to reclaim after use and have long lifespans. These structures act very simply as a filter for harmful meta ions; a microscopic filter for atoms.

Source: Moronshing et al. (adapted)
Furthermore, these NCFS work on a range of pH 2-13, with no significant drop across this large range. This means it can perform well on most all samples of water, and effectively decontaminate water safely. NCFs are also synthesized in a very simple fashion, requiring only minor modifications to an already common nanomaterial known as DFNS (dendritic fibrous nanosilica).
While there is certainly room for expansion into purification of other heavy metals that pollute water supplies, such as lead, this is a very promising step forwards!
-Griffin Bare