With everyone staying at home due to recent events, a common struggle is finding ways to pass the time. You remember having a box of LEGO bricks lying around, but you think to yourself “Building with LEGO? Nah, that’s just a kid’s toy!”. However, LEGO bricks are more than just a construction toy, they are also a technological marvel both in manufacturing and function.
The LEGO group first patented their iconic LEGO brick design in 1958 in Billund, Denmark. Modern LEGO bricks are made of ABS plastic, a copolymer of acrylonitrile, 1,3-butadiene and styrene, which is formed into its distinct brick-like shape using the injection molding process. The molds are designed to produce LEGO pieces accurate to up to five-thousandth of a millimeter (0.005 mm), which is around half the thickness of a human hair. This results in a piece defect rate of 0.0018%. In other words, the LEGO manufacturing process produces 18 defective pieces out of every 1 million total pieces produced.
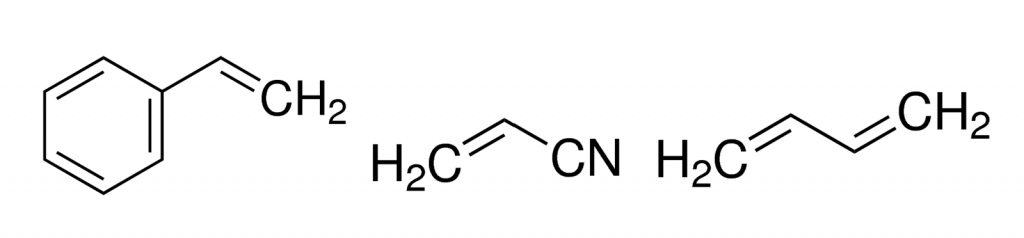
Left to right: Structures of styrene, acrylonitrile and 1,3-butadiene, the main components that make up the plastic used to make LEGO bricks. Source: Sigma-Aldrich
LEGO bricks have become an iconic construction toy due to the endless building possibilities they present. LEGO bricks are connected together through round nubs on top of the brick, known as studs, to tube-based cavities on the bottom of the brick. For instance, six LEGO bricks that are two studs wide by four studs long, commonly referred to as a 2×4 brick, can be combined in 915,103,765 unique ways. This number was determined computationally by mathematics professor Søren Eilers from the University of Copenhagen in 2005.
- Top view of a 2×4 LEGO brick
- Bottom view of a 2×4 LEGO brick
- One way of combining six 2×4 LEGO bricks
- Another way to combine six 2×4 LEGO bricks
- Another way to combine six 2×4 LEGO bricks
(Photo credit: Mark Rubinchik)
Although a LEGO brick may seem like a simple piece of plastic, there is a lot more to it than meets the eye. Next time you’re looking for something to do, why not pull out some LEGO and see how many combinations you can make!
-Mark Rubinchik






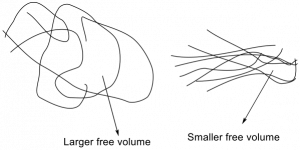 Figure 3. A flexible polymer system due to a large free volume (left). A rigid polymer system due to a small free volume (right). Polyurethanes would resemble the small free volume system due to intermolecular hydrogen bonding.
Figure 3. A flexible polymer system due to a large free volume (left). A rigid polymer system due to a small free volume (right). Polyurethanes would resemble the small free volume system due to intermolecular hydrogen bonding.