Resonance structures are a tough concept for chemistry students to understand, and the way they’re being taught has a significant impact on how well they retain the concept. A pair of researchers from the University of Nebraska and the University of Virginia’s Chemistry departments show that explaining the limitations of resonance drawings gives a better understanding than explaining the benefits.
The study, published March 13th of this year, took 180 students across 2 different organic chemistry classes with 2 different professors. The first professor focused on highlighting how accurate and beneficial resonance drawings are towards the understanding of the deeper chemistry. The second professor focused on the limitations and shortcomings of resonance drawings, and this approach gave a more accurate understanding of the underlying chemistry.
Reliability of Findings
Quantifying a student’s understanding of a concept is no easy task, so the researchers had to cover multiple facets of understanding. They asked students three questions relating to the underlying understandings of resonance contributors of enolates; written description of resonance hybrids, draw the resonance hybrids, and predict the carbon-oxygen bond length. The answers students gave are graphed below, and show that students in Professor 2’s class had a better understanding of all 3 concepts.
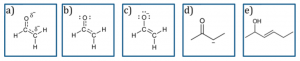
Examples of answers for students asked to draw an enolate resonance hybrid: (a) hybrid structure with correct partial charges (correct answer), (b) hybrid structure, (c) major resonance contributor, (d) minor contributor, and (e) example of other structures (incorrect). Source: Xue, D., Stains, M.
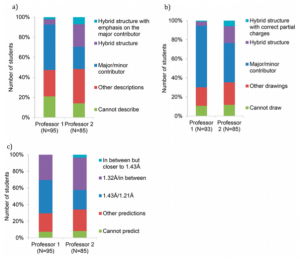
Students’ understanding of resonance hybrids between two professors analyzed through (a) written descriptions of the resonance hybrid, (b) drawings of the resonance hybrid, and (c) carbon-oxygen bond length predictions. Source: Xue, D., Stains, M.
This study did a great job of explaining the ways in which students can misunderstand resonance hybrids, and the pitfalls that professors need to stray students away from. However, teaching styles are not quantifiable, only qualitative observations can be made. This makes any differences possibly anecdotal. Further uncertainty is introduced by limiting the categories that a given student’s answer could fall under. This could “round up” some students to appear to understand more than they do, or vice versa. Professor 2 also had 8 fewer students than Professor 1. This means Professor 1 had 109.4% the number of students that Professor 2 had, making the population sizes of the two groups significantly different in size.
Despite these possible problems with the methodology and certainty of this research, I think we can all empathize with how tough these concepts were at first. And to lighten the tone, I’ll share this analogy that Professor 2 used in his class to help his students learn. Hope it brings a smile to your face!
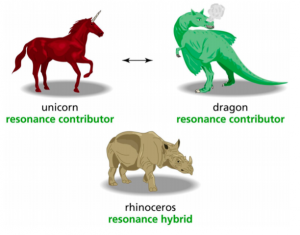
Analogy used by Professor 2 to teach about resonance. Source: Xue, D., Stains, M.
- Griffin Bare
