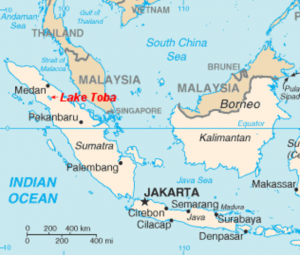
If you trekked through the mountains of northern Sumatra you might see one very long, serene lake and a sizable oblong island in its middle. The whole thing looks very peaceful until you realize it came to be through a cataclysm of unimaginable proportions.
Around 74,000 years ago in those mountains, a supervolcano was brewing world changing fires that erupted suddenly and violently.
Around 2800 km³ of material was released in an outpouring of hot gasses and volcanic ash that wreaked havoc on an area of approximately 20000 km² around the volcano. This means that an area the size of modern day Slovenia was vaporized, since the temperature of these initial volcanic ejections can reach upwards of a 1000 °C. Ash deposits are found today as far away as the eastern parts of Africa.
Scientists say that a global winter ensued which devastated human populations around the
world, leaving only a few surviving groups in the denvse forests of Africa. Investigations of the human genome have revealed that modern humans within Africa possess much higher genetic diversity than do humans outside of Africa. Evidence from studies of mitochondrial DNA suggests that humans passed through what is known as a genetic bottleneck: a profound decrease in genetic diversity and a fall in the total human population to a few thousand breeding pairs. This is known as the Toba catastrophe theory.
In recent years, more evidence and more precise dating of the eruption timeline paints a different, more interesting picture. Some researchers posit that human beings didn’t just survive, but even thrived after the eruption. We have found ancient tools on African coastlines both above and below the layer of ash deposited by Toba. Interestingly, a greater number and diversity of tools seem to be found after the explosion.
The climate data is complicated. Studies of ice cores reveal that the earth cooled drastically right after the eruption and there followed several years of lower precipitation. But other data suggest that certain species of plants recovered quickly after the event, and fossil evidence indicates that orangutang populations close to the area didn’t drop much either.
Scientists now believe that Toba didn’t release a huge amount of sulfuric acid aerosols as compared with other volcanoes, which contribute significantly to the cooling effect. The chemical composition of the magma might have been such that proceeding winter was substantially less intense, and it is theorized that a lot of water vapor might have been released which actually would have counteracted the cooling effect.
The mystery is far from solved. A recent 2021 study looked at the effect of the eruption on the ozone layer, and concluded that it would have made possible for a lot of dangerous UV light to reach the surface of the earth, killing off a lot of humans in the process. Then there is the glaring observation that Neanderthals went extinct only 30000 years ago, long after Toba.
So, there are a lot of unknowns. The only thing we know for certain is that earth will see another Toba at some point in the future, and the fallout could be much, much worse.