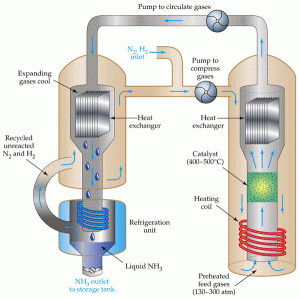
The Haber process is the nitrogen fixation reaction of nitrogen gas and hydrogen gas using an iron or ruthenium catalyst, under high temperature of 500c and pressure of 250 atmospheres (Clark 2002). The goal of the process is to convert nitrogen and hydrogen from the atmosphere to ammonia (NH3). Ammonia is very important to fertilizer industry. Approximately 80% of ammonia is used as fertilizers and it helps provide increased yield of crops (Clark 2002). One of the top challenges for organometallic chemistry is to find a way to catalytically produce ammonia from nitrogen at room temperature and ambient pressure.
Although nitrogen gas makes up 78% of Earth’s atmosphere, it is chemically unreactive under normal condition due to the triple bond between two nitrogen atoms. High energy is needed to break these bonds (Ausetute 2012). Nitrogen fixation occurring in plants is slow. With the help of the Haber process, nearly 100 million tons of ammonia fertilizer is produced every year, yielding taller and healthier crops.

N2 (g) + 3 H2 (g) ⇌ 2 NH3 (g) (ΔH = −92.22 kJ*mol−1)
The production of ammonia is exothermic. There are 4 molecules on the left-hand side and only 2 on the right-hand side. The reaction is reversible and will reach equilibrium when the rate of forward reaction equals the rate of reverse reaction.
If we increase the pressure the system will favour the reaction, producing more ammonia. This is Le Chatelier’s Principle. To get more ammonia produced, high pressure, i.e. 250 atmospheres is needed. Increasing the pressure also speeds the reaction. Under high pressure, molecules are brought closely together and able to contact with the surface of the catalyst. The higher the pressure, the faster the rate of a gas reaction will be.
The catalyst, on the other hand, does not play a role in the position of chemical equilibrium. It lowers activation energy and hence increases the reaction rate. Osmium and ruthenium were used as catalysts at first and iron catalyst is used more often now (Ausetute 2012). Iron is more active so less pressure is needed.
In UBC, Dr. Martinez and her research group are trying to find a way to obtain hydrogen and nitrogen from air and make ammonia without consuming too much energy.
Our video, discussing nitrogen, ammonia, the Haber process and its impact on the fertilizer industry.
https://www.youtube.com/watch?v=T6S02rwVUp8&feature=player_embedded
Our podcast. A brief overview of the environmental impact of the use and production of nitrogen fertilizers.
Audio clip: Adobe Flash Player (version 9 or above) is required to play this audio clip. Download the latest version here. You also need to have JavaScript enabled in your browser.
References:
Clark,J. 2002. http://www.chemguide.co.uk/physical/equilibria/haber.html (accessed Apr. 1 2012)
Asecute. 2012. http://www.ausetute.com.au/haberpro.html (accessed Apr. 1 2012)
Pictures:
http://www.nasdaqinsurance.com/wp-content/uploads/2011/07/crops-insurance.jpg