When someone talks about negative temperature, what usually comes to your mind first? Probably the cold winters with endless snow causing traffic problems in the city if you read temperature in Celsius, or the frozen wasteland of Antarctica if you read temperature in Fahrenheit. But if you are studying thermodynamics and read temperature in Kelvin, then negative temperatures would be very puzzling to understand.
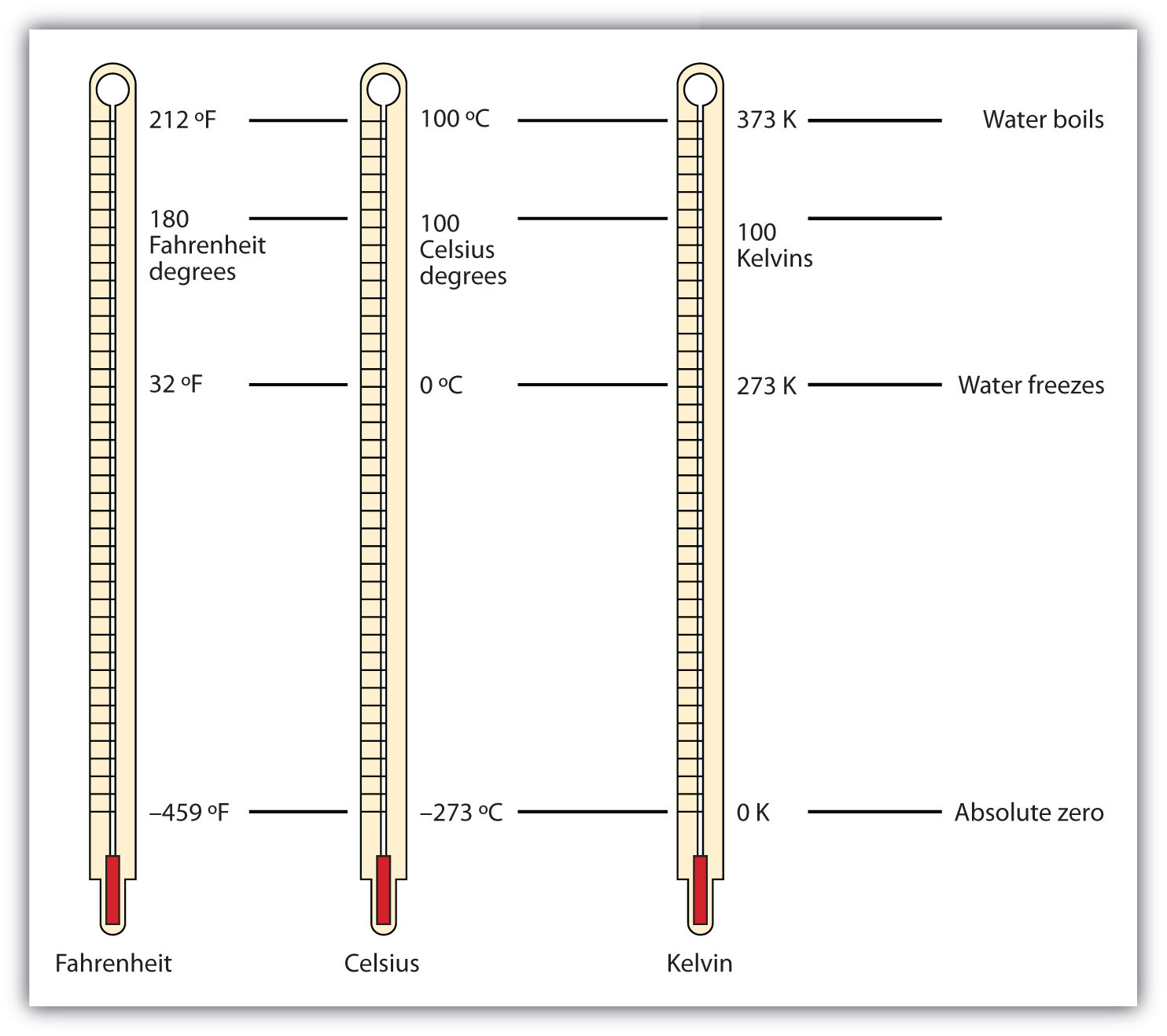
Comparison between Fahrenheit, Celsius, and Kelvin scales
On the Kelvin scale, the lowest possible point is 0 K, absolute zero, where everything ceases to move because of the lack of energy, and the highest possible point depends on which model or theory you coincide with, but for simplicity sake, we’ll call this point absolute hot. Temperature is measured based on the following formula T = dU/dS, where S is entropy and U is internal energy, and if U is zero, that means temperature must be zero as well. Already, we see that the temperature cannot be negative because a system having negative energy is confusing and we don’t deal with it in our daily lives. Although scientists have been able to create negative temperature in the past, recently, according to Nature, Schneider and his fellow researchers published an article about managing to produce a stable substance at negative temperatures using a quantum gas, lasers and magnetic fields.
Under positive temperature systems, atoms too close to each other would repel one another due to the charges the subatomic particles possess. However, once Schneider and his team switched the magnetic field, it caused the atoms to attract one another and be from their lowest possible energy state to the highest. “It’s like walking through a valley, then instantly finding yourself on the mountain peak,” says Schneider. Under normal circumstances, the atoms’ configuration is unstable, but maintaining this with the help of laser fields has caused the atoms to be just below absolute zero, a few billionths to be exact.

One of the properties of negative temperature molecules: more atoms occupy higher energy states
Having negative temperature is not only a strange concept, but atoms reaching this state elicit some strange properties as well, some of which are from dark energy. Investigating its properties will help cosmologists out with figuring out the secrets of dark energy and the Universe. Maybe more innovations may arise after further research has been done, such as incorporating superconductivity in our lives.
For more visuals and information, you can watch the following Youtube video:

Derrick Lee

4 responses to “Below Absolute Zero?”