Could you imagine a world where your smartphone could charge to full in mere seconds? Or be able to take that same battery and charge it over thousands of times without it losing its battery life? And even better, take that same battery once it’s done and be able to throw it into your compost without worrying of needing to dispose harmful chemicals? The battery that sounds too good to be true could be already here, which may well be the next revolutionary solution to all your electrical needs.
These graphene supercapacitors combine the best of both worlds: a battery’s ability to hold high amount of energy, and capacitor’s ability to produce a high output, which in consquence means a fast charging time. Graphene supercapacitors work by using two metal plates seperated by an electric double-layer, approximately 1 molecule thick. Capacitance also increases as the surface area of the two plates increase, while the electric double layer thickness decrease. This makes graphene an optimal material to minimize this distance in the double layer.
There are two big challenges which we face with graphene supercapacitors. One is producing a high enough energy density. They often produce only 1/5th to 1/10th of the energy of an electrochemical battery, like most of today’s lead based batteries. Second, is to produce them in large quantities which are commerically ready.

A diagram of electrochemical cell. These are similar to how common batteries work. By creating an electrochemical potential between two chemicals, batteries utilize redox reactions to create electric energy to power machines.
Image taken from: http://www.mymcat.com/wiki/Electrochemical_Cells_Introduction
That’s when, like all great science, accidents can happen for the better.UCLA Professor Ric Kaner, and graduate student Maher El-Kady stumbled upon a way to make industrial grade graphene supercapacitors, with a common 21st century household machine: a DVD lightscribe burner.

With this new method of producing one atom-thick graphene supercapacitors (which are nicknamed ultra-capacitors for their superior properties), this new way of manufacturing allows them to produce a similar if not superior energy density to electrochemical batteries. Kaner and El-Kady were able to produce 100 micro-supercapacitors in less than 30 minutes.
What is brilliant about this discovery is how remarkably thin the power source actually is. With a thickness of less than 100 nanometers, the applications of this battery are virtually limitless, and allow flexible technology to become a near future reality.
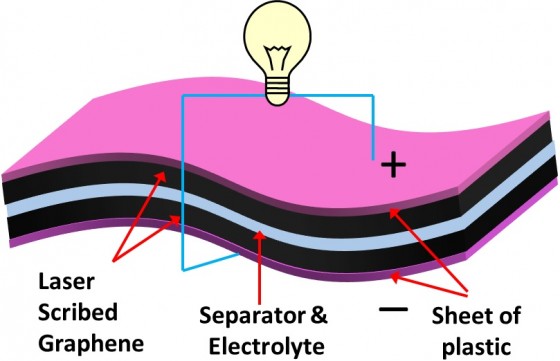
With less than 100 nanometers in thickness, UCLA researchers produce one of the thinnest graphene supercapacitors yet, using Lightscribe DVD burners as a way to manufacture these micro-supercapacitors.
Photo taken from: http://www.greenprophet.com/2013/03/supercapacitor-graphene-maher-el-kady-breakthrough-ucl/
So before you buy the next best smartphone, remember that these supercapacitors are only just a short wait in the future, which might mean that your next phone could be something as futuristic and as flexible as the concept phone, Nokia 888.
