I remember when I was a kid (or even now), one of my all time favourite snack is strawberry flavoured Jell-O (or jelly). Not to mention, making it was so easy and so much fun, as the strawberry aroma would fill the kitchen.
Scientists at the University of Cambridge, led by Peter St George-Hyslop used nematode worm C. elegans as a model for amyloid lateral sclerosis (ALS) and frontotemporal dementia to study the physical properties of FUS, an essential RNA-binding protein in the body. The behaviour and physical properties of FUS can be closely compared to that of jelly. All RNA-binding proteins have two common domains: one for binding RNA and the other where the protein appears to be unfolded. It is at this unfolded region that the FUS undergo a process of reversible ‘phase transition’, which closely resembles the formation of jelly.
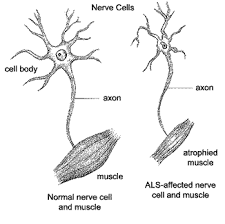
One common characteristic of all neurodegenerative disease is the irreversible accumulation of misfolded or mutated proteins aggregates in the brain, which as a result causes damage to the brain and disrupts communication between brain cells. FUS is one of many types of RNA-binding proteins that is essential to the brain. It is essential in the regulation of protein synthesis, with functions in the nucleus and cytoplasm of a cell. However, the accumulation of mutated FUS and other associated proteins is also the underlying cause of the neurodegenerative diseases such as ALS and frontotemporal dementia. Until recently, the significance and how FUS proteins affects the development of these neurodegenerative disease has been unclear.

[Video courtesy of C.D. Net]
FUS starts out as soluble monomers (like the initial powered-form of jelly), and forms distinct localized accumulations. As it further condenses, a thick gel-like hydrogel structure is formed (like the formation of jelly after it cools in the fridge). This process can be reversible (like warming and cooling jelly repeatedly). Furthermore, during these transitions, RNA and proteins are continuously released from protein assemblies (like suspended fruits in the jelly as it is re-warmed and re-cooled).
The above processes are beneficial because it allows the cells to accumulate cellular machinery in a confined three-dimensional space (with no cell membrane required) when needed to perform key tasks, but also disassemble when not needed. In addition, it is also faster and less-energy costly compared to the formation of a membrane-bound vesicle.
Although FUS is able to carry out vital cell processes by interchanging between different states, “this essential property also makes them vulnerable to forming more fixed structures if mutated, disrupting their normal function and causing disease” says Professor St George Hyslop. Mutation of FUS causes it to over-condense and become a thick fibrous gel, irreversibly trapping the essential RNA and proteins required for protein synthesis. It is the accumulation of misshaped FUS and other RNA-binding proteins that causes serious neurodegenerative diseases. However, further research and understanding of what are in these assemblies can bring us one step closer to curing ALS and other neurodegenerative diseases.