You may remember from biology textbook that we can edit genetic material using restriction enzymes, a DNA-cutting protein structure. Using this we can learn about gene functionalities, search for disease treatments or increase yields of crops. Controversially, it also made unnatural creations possible. However, different gene targets require different enzyme structures, just like to open different doors you need different keys, and making these “keys” turns out to be complicated, expensive and time-consuming, which might have kept most of the “fantasies”, such as “super human” or other wired things, away from reality for now for better or worse.
However, what if all the “doors” also have the same type of “digital locks”, and all you need to change is the password? CRISPRs (clustered regularly interspaced short palindromic repeats) turns out to be this “lock”. It contains repeated sequences sandwiched with spacers (unique genetic information) in between. The spacers are external virus genes kept in bacterias and served as the “criminal records” so that when invasion happens again, the bacteria can send an “army” of gene-cutting enzymes, called Cas9, to cut the recognized viruses’ DNA apart.
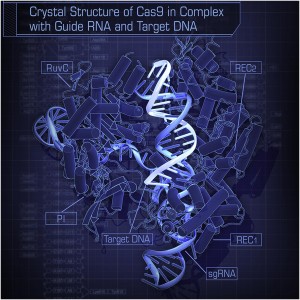
“Crystal Structure of Cas9 in Complex with Guide RNA and Target DNA” by Hiroshi Nishimasu, F. Ann Ran, Patrick D. Hsu, Silvana Konermann, Soraya I. Shehata, Naoshi Dohmae, Ryuichiro Ishitani, Feng Zhang, and Osamu Nureki – Crystal Structure of Cas9 in Complex with Guide RNA and Target DNA http://dx.doi.org/10.1016/j.cell.2014.02.001. Licensed under CC BY-SA 3.0 via Commons
In 2012, scientists have proven that instead of making restriction enzymes for different genes, we can simply replace these “criminal records” and use the same Cas9 “armies” to cut desired genes with much less time and cost. You can find the details in this following video.
 [by McGovern Institute for Brain Research at MIT]
[by McGovern Institute for Brain Research at MIT]
Nature , Science and many major media have unanimously deemed CRISPRs to be a revolution. Radiolab has discussed its ethical controversies as if the realization of the technology is right around the corner. It has been portrayed as such a simple and approachable method that people even claim to provide CRISPR DIY kit for experiments at home.
However, I think despite these attentions, the reality is that we are still far from ready to harness CRISPRs. Although many researches has already been using it to edit genes in a variety of bacterias, plants, and animals within a short 3-year period, few studies focused on the limitations and functions of CRISPR itself. Some research was done too early without waiting for the technology to mature, such as the research using human embryos, which has resulted in a less satisfactory result. Some researchers commented that CRISPR can be less accurate than expected and may not be the most efficient and cost-effective solution for certain problems on Quora. Scientist Konstantin Severinov on Quanta Magazine also pointed out that it is still unknown whether defending invaders is the major usage of CRISPRs since many of these spacers appear to be genes of viruses that are long gone. It might be a good time now to slow down and maybe build the technique from ground up concretely first.
by Sainan Liu