Source: CultBeauty Facebook page
As the winter solstice approaches, you may have continually been nagged by a friend or a loved one to “Put on some hand cream!” or to “Always put on moisturizer!”
As temperatures drop lower, and heating systems cause indoor air to become drier, our skin also suffers. Dry skin is more than just itchy and flaky skin: if not properly cared for, dry skin can lead to skin cracking, bleeding, infection, and even eczema. Simply put, moisturizers work to prevent these symptoms by maintaining the skin’s barrier and preventing water loss.
So, how exactly are these aesthetically-packaged creams able to do that?
Cause of Dry Skin
Source: compoundchem.com
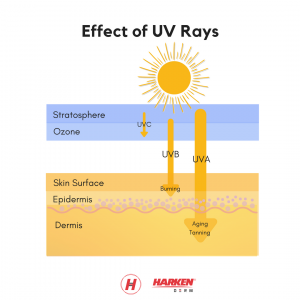
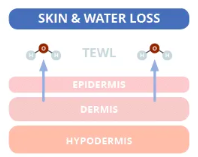
First, we must understand the cause of dry skin. The skin’s middle layer, the dermis, is mainly responsible for storing water. Skin dries out through a normal process called trans epidermal water loss (TEWL). Essentially, TEWL is when water passes from the dermis through the epidermis and evaporates from the skin’s surface. The average TEWL in humans is about 300–400 mL/day, but this number is affected by environmental factors (e.g. cold weather and dry air).
Active Ingredients in Moisturizers
Moisturizers aim to minimize TEWL by preventing water evaporation and replenishing moisture in the epidermal layer. Moisturizers are composed of three main active ingredients: occlusives, emollients, and humectants. Most products today feature some combination of these actives, in varying proportions.
Occlusives
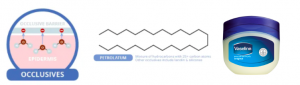
Petrolatum, or Vaseline, a popular occlusive. Source: compoundchem.com
Occulusives are large, heavy molecules which contain long carbon chains that repulse water. They form a physical barrier over your skin’s outer layer which water cannot penetrate, effectively stopping evaporation. A popular example is Petrolatum or Vaseline. Despite its effectiveness, this ingredient tends to be sticky and greasy thus is commonly formulated with the other actives in today’s moisturizers.
Emollients

Castor oil, a popular emollient. Source: compoundchem.com
Emollients are structurally similar to occlusives: these molecules also contain long, fatty carbon chains like stearates and castor oil. To fully understand how emollients work, the skin’s outer layer, the epidermis, must be discussed.
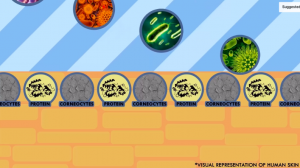
Visual representation of the stratum corneum. Source: YouTube
The epidermis consists of sub-layers: the outermost layer, called the stratum corneum, is composed of dead skin cells (corneocytes) and proteins that jointly forms a protective barrier between your body and external microbes/toxins. In dry conditions, the proteins denature thus create gaps in the skin’s protective barrier. Instead of coating your skin as occlusives do, emollients are able to penetrate into your skin’s outer surface to fill in these gaps. This ultimately prevents TEWL and additionally improves the appearance of skin.
Humectants
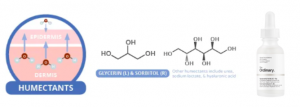
Hyaluronic acid, a popular humectant. Source: compoundchem.com
In contrast to occlusives and emollients, humectants are hydrophilic, meaning that they attract water. They draw water from the dermis to the epidermis; this helps to get newer, moist skin cells toward the outer layer of the skin thus reduce dry skin flakiness. Additionally, humectants stimulate body’s natural production of ceramides, which reduce TEWL by acting as emollients (i.e. filling gaps in the skin’s barrier). At high-moisture conditions (humidity > 80%), humectants are able to attract more water from the environment into your skin.
So, the next time someone nags you to “put on some moisturizer!” this winter season, listen to them! And be grateful that someone cares about your (and your skin’s) well-being!
Written by Ambi Atienza