A team of researchers at the National Institute of Environmental Health Sciences (NIEHS) have found a promising antiviral in the strangest place – a protein called AEG12 found in the gut of mosquitos. This recent study lead by Dr. Alexander Foo found that AEG12 strongly inhibits the family of viruses that cause Yellow Fever, Dengue, West Nile, and Zika, and that the protein may also inhibit their distant relative: coronaviruses. Although the virus-killing AEG12 protein sounds promising, it may still be a long time before its safe to use in humans.

The Aedes aegypti mosquito, which can carry viruses such as Yellow Fever, Dengue, and Zika (Image source)
Foo and His Findings
Scientists have long been on the hunt for effective antivirals, and researchers like Dr. Foo have been making great progress in the field. Foo’s recent study showed that AEG12 was effective at killing flaviviruses (such as Dengue and Zika) but was less effective at killing coronaviruses. Using methods and software tools for understanding how proteins work and specialized laboratory techniques to study viruses, the team was able to piece together how AEG12 is such a successful virus-destroyer.
Flaviviruses are enveloped: meaning that each virus particle is surrounded by a membrane. The infection process, in the video below, shows how the virus must first attach to the surface a human cell (0:28) and then fuse its membrane with ours to get inside (1:12).
Essentially, the AEG12 protein carries little membrane pieces of its own, which are different from the type found in the virus’ membrane. When it comes across a virus, AEG12 trades its pieces for the virus’. Once enough chunks are swapped out, the virus’ membrane becomes unstable and can’t fuse with our cells: effectively “killing” the virus.
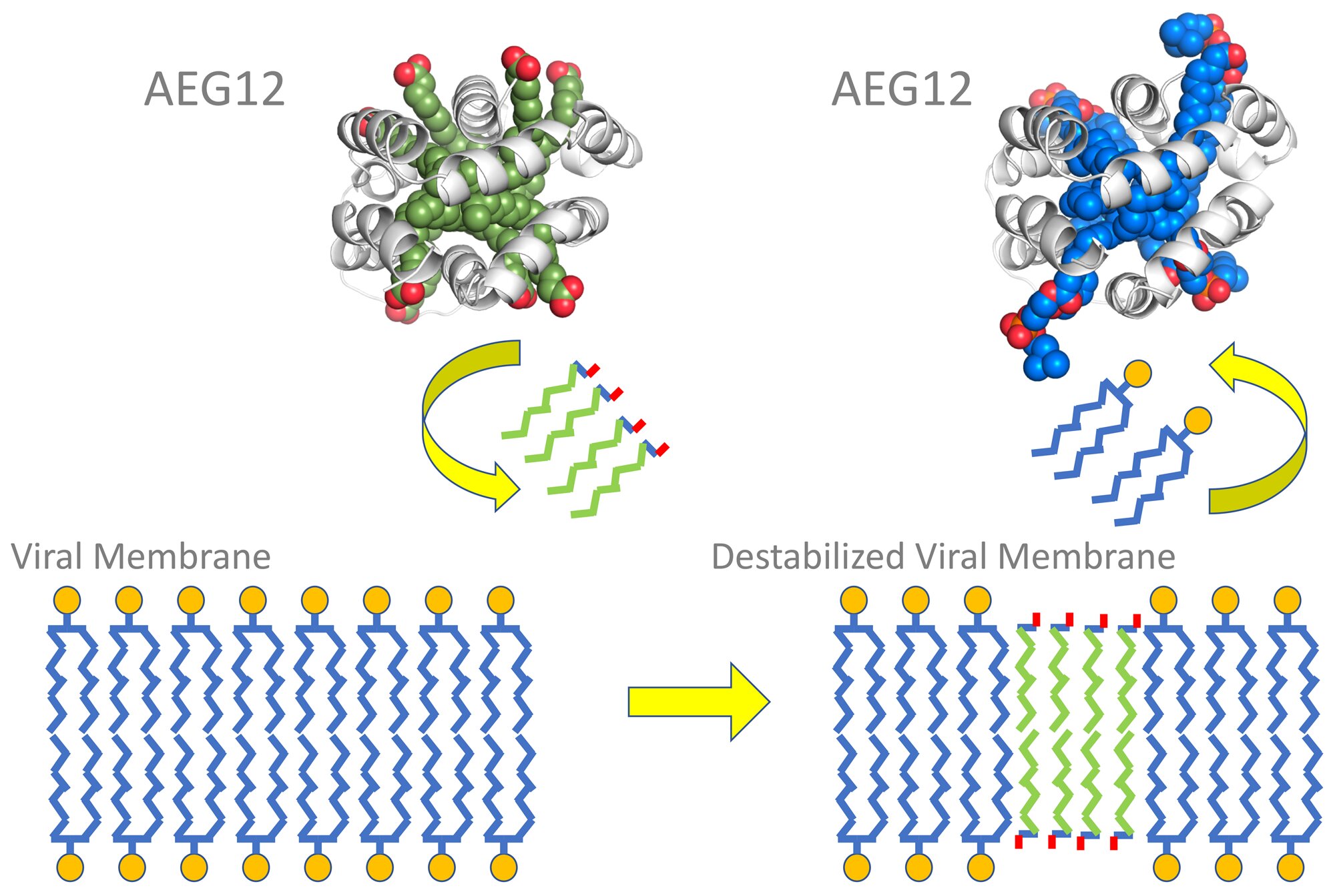
The AEG12 protein swaps out viral membrane pieces for unstable pieces (Image source)
Even though AEG12’s virus-killing abilities sound promising, Foo’s team found that AEG12 breaks apart red blood cells. Although useful to a mosquito that needs to digest a blood meal, use of AEG12 in humans would lead to a very serious blood disorder that could be fatal if left untreated.
There is Still Hope
So, AEG12 isn’t safe for use in humans… Yet. With some clever solutions and careful bioengineering, further research could find a way to modify AEG12 so that it targets viruses and ignores human cells. The development of a drug or molecule effective against enveloped viruses could save lives across the globe – addressing not only the headlining COVID-19 pandemic, but the ongoing epidemics as well.
-Maya Bird

 When to get tested for COVID-19
When to get tested for COVID-19 Abbott’s ID Now COVID-19 Test
Abbott’s ID Now COVID-19 Test

 A Typical Nasal Swab
A Typical Nasal Swab