Thoughts on T-GEM:
Khan (2007) identified model-based inquiry as a "dyanmic, recursive process of learning by changing one’s mental models while inquiring about phenomena." GEM was a pattern of teacher-student interactions noticed during a study of model-based inquiry in the classroom. This pattern consisted of helping students to construct a hypothesis/model from collected data(generation), creation and application of a hypothesis from the data (evaluation), and then with prompts and guidance the revision of the model (modification). Since today’s learners expect to use media during their learning (Beyers, 2009) the natural progression of model-based-inquiry is to facilitate it with technology. Affordances associated with technology include, accurate record keeping, visualization and testing of processes, which are either unobservable (too small, fast, or far) or too dangerous (Edelson, 2001) and the capacity for dynamic presentation. Technology can also allow for asynchronous inquiry, such that the learners are able to continue the process outside of the classroom, enable a more flexible learning pattern to develop. In the follow section I attempted to create a T-GEM model to assist learners with building an understanding of the mechanisms behind chemical bond formation.
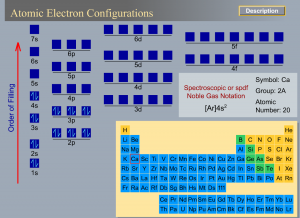
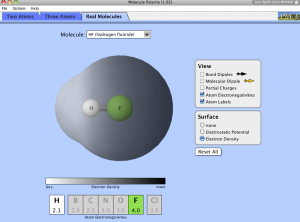
Issue:
An inaccurate understanding of how and why elements form different types of compounds (ionic & molecular) due to electro-negativity and electron configuration.
Background:
Students are provided with the knowledge that atoms consist of electrons, which exist in different set energy levels and that only a certain number of electrons can exist in particular shells. Electrons are responsible for creating bonds.
T-GEM:
Technology:
Generation-I:
- Use Atomic electron configuration to identify the number of electrons and the patterns in the first 20 elements.
- Students are then asked to predict the electron configuration for randomly selected elements and explain their rationale.
Evaluation-I:
- Propose explanation of how electrons configuration lead to the formation of compounds.
- At this stage questions regarding where electrons are “moving to” and type of compound being formed would be relevant.
- As a teacher I would encourage for discussions and consensus among peers for explanation.
Modification-I:
- Based on the different possible combination students achieved, have them account for why metals don’t form compounds with one another and to account for ionic versus molecular compounds.
Generation-II:
- Students would use “Real Molecules” from PhET to collect data on electron density and electro-negativity and identify patterns and trends.
- Prompts and questions about location of electron density could be used to help them identify patterns.
Evaluation-II:
- Students create a hypothesis as to why electron density and electro-negativity are tied to electron configuration.
- Predict the characteristics of randomly selected compounds and discuss findings and explanations with peers.
Modification-II:
- Students revision explanation from Modification-I to include observations and conclusion from electro-negativity
Use metalloid compounds to support and justify explanations
Reference:
Beyers, R.N. (2009) A five dimensional model for educating the net generation. Educational Technology & Society, 12(4), 218-227.
Edelson, D.C. (2001). Learning-for-use: A framework for the design of technology-supported inquiry activities. Journal of Research in Science Teaching,38(3), 355-385.
Khan, S. (2007). Model-based inquiries in chemistry. Science Education, 91(6), 877-905.
Khan, S. (2010). New pedagogies for teaching with computer simulations. Journal of Science Education and Technology, 20(3), 215-232.
