Why is visualization a necessary component?
Prior to the introduction of Le Chatelier’s principles students have for the most part be taught that reactions proceed in forward direction (towards products). So this represents a large change in the perceptions of chemical reactions. Observable chemical laboratories are also difficult to carry out, and that static nature of writing equations on a board are at odds with the dynamic nature of Equilibria.
Use and Implications for teaching practices
Use of the virtual chemistry lab can provide opportunities for discovery learning.
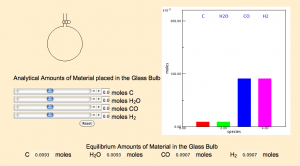 Since the lab contains, instructions for how to explore the concept, students can work in small groups to collect data, evaluate the data to create a hypothesis, and modify the hypothesis through further discourse and experimentation. The teacher then provides guides for collecting data and prompts for analysis and hypothesis creation, which scaffolds the learners construction of the model (Khan, 2007). This scaffolding addresses one of the concerns identified by Srinivasan et al.(2006) that if the learning goals are too high then learning can be impeded.
Since the lab contains, instructions for how to explore the concept, students can work in small groups to collect data, evaluate the data to create a hypothesis, and modify the hypothesis through further discourse and experimentation. The teacher then provides guides for collecting data and prompts for analysis and hypothesis creation, which scaffolds the learners construction of the model (Khan, 2007). This scaffolding addresses one of the concerns identified by Srinivasan et al.(2006) that if the learning goals are too high then learning can be impeded.
What are the affordances?
These include opportunities for students to work in a collaborative, learner-centered approach where they can identify misconceptions and actively formulate new mental models. The first steps are provided so that all students have one set of unified data with which to create initial hypothesis and opportunities (prompted by the instructor as a facilitator) exist for further smaller group explorations. Since it is a web-based tool, students can use the tool asynchronously to further develop their understanding. As Finkelstein et al. (2005) point out, the use of virtual manipulatives is also helping to prevent students attaching incorrect meaning to such things as the physical properties of the chemicals, or making subjective assessments about color change. The virtual labs include interactivity with numerous possible outcomes. In order to create authentic assessments and help with knowledge integration the educator could ask students to predict the outcomes for several scenarios before they actually completed the test.
In schools with adequate resources, the hands on experiments could facilitate discourse about why the results did not necessarily match the expected outcome of the simulation (over simplification of model, impure reactants, etc.). The use of a blended approach would also address students possible perceptions of the simulation as being fake (Srinivasan, 2006) since they could then physically interact with the resources. While difficult to overcome without a blended approach, the use of the simulation can help those students whose only other exposure is via bookwork and sample questions which tell them Equilibria is established to at least formulate an image of molecules shifting. The use of virtual labs must be done with intent and adequate forethought in order for it to be effective in the learning process and then in can promote learning (Finklestein et al. 2005).
Pondering and Questioning
Learners in the MET-program represent a select group of educators with a predisposition to incorporate technology, but aren’t necessarily representative of all teachers. Knowing that simulations, and digital modeling tools can help students to visualize abstract or difficult to observe phenomena, I wonder how to effectively help the “average” teacher incorporate such tools. I also question the actual level of student engagement and critical thinking with such tools, especially for those students who have had very little exposure initially. I see some of these difficulties with my students, who do have some experience and yet still struggled this week to make the “correct” inferences while using simulations from PhET to predict molecular polarity. 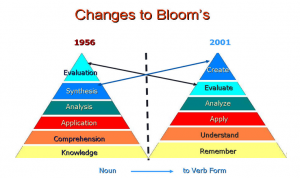 A final observation and question that really standout from the various tools and discussions is that many of the assessments and evaluations still follow into the lower level of Bloom’s taxonomy. So, how can these information visualization tools be used by the students to demonstrate concept mastery and create authentic assessments?
A final observation and question that really standout from the various tools and discussions is that many of the assessments and evaluations still follow into the lower level of Bloom’s taxonomy. So, how can these information visualization tools be used by the students to demonstrate concept mastery and create authentic assessments?
References
Anderson, L. W. and David R. Krathwohl, D. R., et al (Eds..) (2001) A Taxonomy for Learning, Teaching, and Assessing: A Revision of Bloom’s Taxonomy of Educational Objectives. Allyn & Bacon. Boston, MA (Pearson Education Group)
Finkelstein,N.D., Perkins, K.K., Adams, W., Kohl, P., & Podolefsky, N. (2005). When learning about the real world is better done virtually: A study of substituting computer simulations for laboratory equipment. Physics Education Research, 1(1), 1-8.
Khan, S. (2007). Model-based inquires in chemistry. Science Education, 91(6), 877-905.
Srinivasan, S., Perez, L.C., Palmer, R., Brooke, D., Wilson, K., & Fowler, D. (2006). Reality versus simulation. Journal of Science Education & Technology, 15(2), 1-5.
