Hello Team, I hope you enjoy reading my post as much as I will enjoy reading yours!
Please let me know if you have any questions.
Note: In-text citations occur after all consecutive relevant sentences from a single source are completed.
Introduction
This assignment addresses the following:
• The importance and role of definitions in technical writing
• Developing an understanding of how audience and purpose may indicate the need for a definition
• Acknowledgement of differentiation difference in detail level when making a definition
• Choosing the appropriate level of detail depending on the situation
This assignment introduces a relatively complex term from my area of study, pharmacology. A specific reading situation is given, clarifying the purpose of giving the definition. This term is then defined for a non-technical audience using three methods: parenthetical, sentence and expanded definitions, all with professional, yet friendly tone, posted on the team forum.
Term
Parathion
Reading Situation
A farmer is deciding whether or not to use parathion as a pesticide on his corn and wants to know a little more about it.
Parenthetical Definition
Parathion (a pesticide that is toxic to the nervous system) is dangerous to spray on the corn outside your house, as it has the potential to kill humans in addition to bugs.
Sentence Definition
Parathion is a drug of the organophosphate class used as a pesticide. Toxic to humans, the drug acts on the nervous system, blocking breakdown of neurotransmitters and ultimately resulting in death.
Expanded Definition
Parathion
History
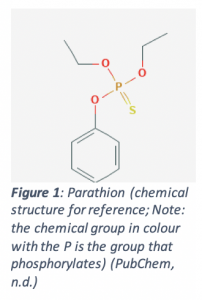
Parathion, a drug of the organophosphate (due to its structure; Figure 1 below) class, was synthesized by Bayer AG (a German pharmaceutical company) in 1947. It was registered by the US as a drug in 1948. Toxic to almost all life, the drug resulted in many human poisonings throughout its history. Half of the world’s pesticide poisonings in the 1970’s were due to parathion, with a reported 1,283 poisonings between 1966 and 1980 (47 deaths and 478 hospitalizations). Contaminations have also resulted in many deaths; for example, one Asian country had 828 cases of poisoning and 106 deaths when the food supply became contaminated with parathion. As a result, it has been banned if not heavily restricted in numerous countries, including Canada (Rubin, Esteban, Hill & Pearce, 2002).
Mechanism of Toxicity
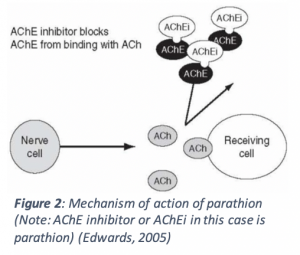
In order to exert its toxic effects, parathion is readily absorbed on the skin, on the lungs (when inhaled) or in the gastrointestinal tract. Its chemical properties allow it to travel to the nerves, where it will act on acetylcholinesterases (AChE), inhibiting their normal function. AChE is responsible for degrading acetylcholine (ACh), a certain type of neurotransmitter, so that it does not overstimulate neurons. In order to act on AChE, parathion must be converted to paraoxon, undergoing a reaction that adds an oxygen to the molecule. Then, the much more reactive paraoxon can phosphorylate (adds a chemical group; refer to Figure 1) an active part of AChE to inactivate it. This will cause ACh to build up, leading to overstimulation of the neuron (Refer to Figure 2 below) (Fukuto, 1990).
Toxicities
Once parathion inactivates AChE leading to buildup of ACh, it will result in an amplification of the usual effects of ACh. Thus, parathion indirectly results in overstimulation of nerve receptors, resulting in exhaustion, blurred vision, headache, weakness and confusion. Pupil constriction, vomiting, abdominal pain, excessive sweating and salivation may also occur. Difficulty in breathing may be present due to lung congestion and respiratory weakness. In severe poisoning one can expect involuntary voiding of the bowel and bladder, convulsions, unconsciousness and muscle spasms. Relaxation of the lungs is generally the cause of death (Robb & Baker, 2020).
Commercial Use
Parathion is commercially used as an insecticide and can also be used to kill ticks and mites. It may degrade due to microbes, soil or sunlight, although sunlight can also convert it into paraoxon, its more toxic form. It is used to control a broad spectrum of pests on alfalfa, canola, corn, cotton, sorghum, soybeans, sunflowers, barley and wheat (but not fruits or vegetables due to its toxicity). In liquid form, it may be applied using aerial equipment. Unfortunately, ecological effects are adverse and widespread, with parathion being very toxic to most animals and aquatic creatures and lasting a long time in soil (but much more rapidly degrading in aquatic environments) (Parathion, 1997).
Reference List
Edwards, F. (2005, December 30). Environmental Toxicology and Health Effects Associated with Methyl Parathion Exposure – A Scientific Review. Retrieved October 01, 2020, from https://www.mdpi.com/1660-4601/2/3/430/htm
Fukuto, T. (1990, July). Mechanism of action of organophosphorus and carbamate insecticides. Retrieved September 29, 2020, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1567830/
PubChem. (n.d.). Retrieved September 29, 2020, from https://pubchem.ncbi.nlm.nih.gov/
Robb, E., & Baker, M. (2020, July 27). Organophosphate Toxicity. Retrieved September 29, 2020, from https://www.ncbi.nlm.nih.gov/books/NBK470430/
Rubin, C., Esteban, E., Hill, R. H., Jr., & Pearce, K. (2002). Introduction–the methyl parathion story: A chronicle of misuse and preventable human exposure. Retrieved October 01, 2020, from https://pubmed.ncbi.nlm.nih.gov/12634136/
Leave a Reply