Introduction:
For this assignment, we were instructed to choose a complex word within our discipline and create three different definitions of this term. The three definitions should be explained so that an audience of non-technical readers, and those with minimal knowledge on the subject, would be able to read the definitions and understand what the term corresponds to. The three definitions should include a parenthetical definition, a sentence definition, and an expanded definition.
Reading Situation:
A professor is explaining the term “electronegativity” to a first year undergraduate student during office hours.
Parenthetical Definition:
Electronegativity (the ability of an atom to attract electrons) is a chemical property of an atom.
Sentence Definition:
Electronegativity is a chemical property of an atom that measures the atom’s tendency to attract shared electrons towards itself.
Expanded Definition:
History
Electronegativity is an important chemical concept of chemistry. The concept of electronegativity was known by many chemists including Avogadro prior to the 20th century (Wikipedia, 2022). However, the concept was not coined electronegativity until Linus Pauling defined it in the 20th century. The term was introduced as an empirical concept, and at the time was defined as “the capacity of a molecule or atom to attract electrons”. Due to it being an empirical concept, electronegativity must be calculated as it cannot be derived using the laws of quantum mechanics (Robles-Navarro et al., 2021).
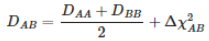
Equation 1 – The equation used to define and calculate electronegativity (Retrieved from Robles-Navarro et al., 2021)
Compare and Contrast – Electronegativity vs Electropositivity
Electronegativity should not be confused with the concept of electropositivity. Electropositivity is the ability of an atom to donate electrons to form cations (positively charged ions) (Vedantu, n.d). This is the opposite to electronegativity, where atoms have the tendency to attract electrons towards themselves. While metals such as Francium and Cesium tend to be not as electronegative, they are fairly electropositive, and will donate electrons to form ionic bonds (a bond formed between oppositely charged ions).
Example – Application to Hybridization
The elements found on the periodic table are sorted based on their electronegativities. As shown in figure 1, the electronegativity increases across a period (from left to right), and decreases down a family (from top to bottom). The most electronegative element is Fluorine, while the least electronegative element is Francium. These values are not constant, and can be manipulated depending on the atom’s hybridization. Hybridization (the atomic orbital interactions between atoms) can affect the overall electronegativity of a molecule. There are three forms of hybridization among atoms, denoted as sp, sp2, and sp3. Atoms that are sp-hybridized will see a greater electronegativity compared to atoms that are sp2 or sp3 hybridized. For example, atoms that are sp-hybridized are 50% s-character, and 50% p-character. In contrast, atoms that are sp2-hybridized are 33.3% s-character, and 66.7% p-character. Atoms that have more s-character tend to hold onto electrons more readily, therefore as the p-character increases due to changes in hybridization, s-character decreases and the molecule becomes less electronegative (Chemistry LibreText, 2021).

Figure 1 – The electronegativities of the elements on the periodic table (Retrieved from Chemistry LibreTexts)
Example – Application to Dipole Moments
As shown in figure 2, the dipole moment (a separation in charge within a molecule) of the molecule can be visualized due to differences in electronegativity. Fluorine is more electronegative than carbon, as seen in figure 1, therefore it tends to hold onto and attracts electrons more readily. This generates a partially negative charge at the fluorine atom, and a partially positive charge at the carbon atom. As a result, a dipole moment, as indicated by the red arrow in figure 2, develops towards the fluorine atom due to the difference in charge.
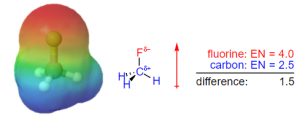
Figure 2 – Analysis of the dipole moment due to electronegativity in CH3F (Retrieved from Chemistry LibreTexts)
References
Electropositivity. (n.d.). VEDANTU. Retrieved January 30, 2022, from https://www.vedantu.com/chemistry/electropositivity
Polar Covalent Bonds – Electronegativity. (2021, September 28). https://chem.libretexts.org/@go/page/31383
Robles-Navarro, A., Cárdenas, C., & Fuentealba, P. (2021). Electronegativity under Confinement. Molecules, 26(22), 6924. https://doi.org/10.3390/molecules26226924
Wikimedia Foundation. (2022, January 27). Electronegativity. Wikipedia. Retrieved January 30, 2022, from https://en.wikipedia.org/wiki/Electronegativity
Leave a Reply