Ethan Fung
ENGL 301
June 12th, 2022
Introduction:
Dear writing team,
This assignment aims to describe the term amino acid in varying levels of detail to non-technical readers. The reading situation will be a third-year chemistry student explaining what research he is working on to his parents. Three descriptions will be given, a parenthetical definition, a sentence definition, and an expanded definition. A few necessary technical terms will be used however, all technical terms will be explained in a glossary.
Word:
Amino acid
Parenthetical definition:
Amino acids (the building block of proteins) are necessary for all living beings to function.
Sentence definition:
Amino acids are a type of biomolecule that behave as the foundation of proteins and are characterized by having an amino1 group, carboxylic acid2 group, and a side chain specific to each amino acid.
Expanded definition:
Etymology
The word “amino acid” comes from the two defining groups present on the molecule. The word “amino” refers to the amine1 group and “acid” refers to the carboxylic acid2 group.
History
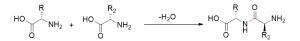
The first amino acid, asparagine was isolated in the early 1800s, by French chemists Louis Nicolas Vauquelin and Pierre Jean Robiquet [1]. Amino acids continued to be discovered throughout the 1800s, however, the function of amino acids in a biological context was not understood. In 1902, Emil Fischer and Franz Hofmeister hypothesized that many amino acids could combine to create proteins via a linkage Fischer termed “peptide bond” [1]. Fischer proposed that the amino group and carboxylic acid group of different amino acids can fuse to create a chain of amino acids called a peptide [1]. Peptides can then become proteins by growing and reaching a functional structure. An example of peptide bond formation between two amino acids is shown in Figure 2.
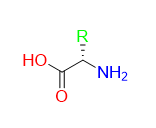
Figure 1: A example of an amino acid, the carboxylic acid group is highlighted in red, the amino group is highlighted in blue, and the side chain is highlighted in green.
Figure 2: Peptide bond (highlighted in red) formation resulting in the loss of water
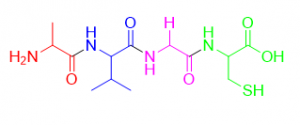
Figure 3: A peptide made from four amino acids: alanine (red), valine (blue), glycine (purple), and cysteine (green).
(Functions) Biological and artificial peptide synthesis:
Amino acids by themselves are useless however, when combined via peptide bond linkage into functional proteins they dictate almost all biological functions. To help visualize this imagine each amino acid as a LEGO® brick. An individual LEGO® brick is useless but when stacked on top of each other they can create magnificent Lego structures. However, unlike LEGO®, forming a peptide bond between amino acids is unfavorable and requires a catalyst/enzyme to catalyze the reaction. In living systems, peptide bond formation is catalyzed by the ribosome, an organelle in the cell which is responsible for making proteins [2]. Outside of biological systems, there are chemical coupling reagents that can incite peptide bond formation allowing for artificial peptide synthesis. In addition, most laboratories use a technique called solid-phase peptide synthesis3 (SPPS) to allow for an easier and more efficient peptide synthesis [3].
Biological and artificial amino acid synthesis:
Like peptides, amino acids can be synthesized biologically or artificially. In biology, there are 20 common amino acids which make up a majority of naturally occurring proteins. In humans, only 11 of the 20 naturally occurring amino acids can be synthesized by the body and are termed non-essential amino acids. Humans must obtain the other nine amino acids through diet. These nine amino acids are called essential amino acids. Artificial amino acids can be prepared in a laboratory through synthetic techniques which allow for total control of all functional groups present on the amino acid. Therefore, from a synthetic perspective, there is an unlimited amount of different amino acids that can be synthesized, each one with its own specific properties.
The future of amino acids in science:
As a result of artificial amino acid synthesis, many scientists are interested in making modified peptides and proteins from novel amino acids. These proteins may contain desired functionalities not available in nature which may contribute to advances in medicine and biotechnologies. Additionally, there are many important natural proteins that have not yet been synthesized artificially. Overall, the synthesis of different amino acids, peptides, and proteins will continue to be a trending topic in the world of biochemistry and chemistry for years to come [4]. The peptide therapeutics market was reported to be valued at 39.6 billion dollars in 2021 and is expected to grow in the following years [4].
Glossary:
- Amine/amino: A functional group in organic chemistry, composed of a nitrogen bonded to 3 other elements.
- Carboxylic acid: A functional group in organic chemistry, composed of a carbon double bonded to an oxygen and an alcohol (OH)
- Solid-phase peptide synthesis: The use of a solid support, usually in the form of glass beads to guide peptide synthesis. It allows for the use of a machine called a peptide synthesizer which can automatically build peptides through a user inputted sequence and amino acid reservoirs.
Works Cited:
- Vickery, Hubert Bradford., and Carl L. Schmidt. “The History of the Discovery of the Amino Acids.” Chemical Reviews, vol. 9, no. 2, 1931, pp. 169–318., https://doi.org/10.1021/cr60033a001.
- Ribosome. Genome.gov. (n.d.). Retrieved June 6, 2022, from https://www.genome.gov/genetics-glossary/Ribosome#:~:text=A%20ribosome%20is%20an%20intercellular,that%20fold%20to%20form%20proteins.
- Merrifield, B. (1986). Solid phase synthesis. Science, 232(4748), 341–347. https://doi.org/10.1126/science.3961484
- Global peptide therapeutics market size, share report, 2030. Global Peptide Therapeutics Market Size, Share Report, 2030. (n.d.). Retrieved June 5, 2022, from https://www.grandviewresearch.com/industry-analysis/peptide-therapeutics-market#:~:text=The%20global%20peptide%20therapeutics%20market,6.4%25%20from%202022%20to%202030.
Leave a Reply