Introduction:
For this assignment we are tasked with picking a relatively complex term in a field which we have experience with. For this term, we are to compose a parenthetical, sentence and expanded definition to an audience of non-technical readers based on a simulated scenario of our choosing. The objectives of the assignment are to appreciate the role of definitions in technical writing, understand how various audiences and purposes indicate the need for definitions, differentiate between the different types of definitions, and selecting the right level of detail according to the situation.
Reading Situation:
A pharmacist is explaining to a patient the concept of a bioequivalent medication to a patient.
Parenthetical Definition:
Bioequivalent medication (medications that contain the same active ingredient) can be used interchangeably and still achieve the desired response.
Sentence Definition:
Bioequivalent medication is two medication that are chemically and biologically equivalent with both containing the same active ingredient. Bioequivalent is often seen when comparing generic and brand name medication (acetaminophen vs Tylenol) both can be used to treat the same medical condition, and both have the same effects.
Expanded Definition:
What is Bioequivalent Medication
Bioequivalent medication is two or more medication that contain the same active ingredient and can be used interchangeably.
Why did Bioequivalence Develop?
Bioequivalent medications are historically created to offer a less expensive option to the patient. When a medication is created there is a large investment in research and development, this results in a new brand name medication. After a few years companies are allowed to copy the formula of a brand name medication and create bioequivalent generic medication with the same medical ingredient and the same effect in the body (Midha and McKay, 2009).
How is Bioequivalence Determined
Bioequivalence is determined in four steps:
Step 1) The original brand name medication is made.
Step 2) After the allotted time has passed other companies copy the brand name medication.
Step 3) From here the two medications, brand name and generic, have the amount in a person’s body measured over a set duration of time (Figure 1.) to ensure that these amounts are the same (Nation and Sansom, 1994).
Step 4) Once these amounts are deemed the same, the two medications are said to be bioequivalent of one another.
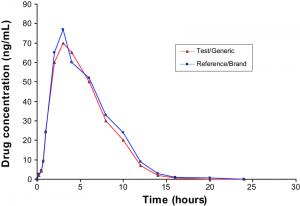
Figure 1. Comparison of Brand and Generic medication in terms of the amount of medication in the body over time (Mastan et al., 2011).
Bioequivalent Products Available Today
Almost every medication has bioequivalent options. Examples of bioequivalent medication are seen in common cold and flu medication; Advil is a common brand name used to treat cold and flu, its generic alternative is Ibuprofen and these medications are considered bioequivalent. Furthermore brand name Tylenol often used to treat a fever has a generic name of Acetaminophen and these two medications are bioequivalent. In both instances the generic has the same effect as the brand and is offered at a fraction of the price.
References:
Mastan, S., Latha, T. B., & Ajay, S. (2011). The basic regulatory considerations and prospects for conducting bioavailability/bioequivalence (BA/BE) studies–an overview. Comp Eff Res, 1, 1-25.
Midha, K. K., & McKay, G. (2009). Bioequivalence; its history, practice, and future. The AAPS journal, 11, 664-670.
Nation, R. L., & Sansom, L. N. (1994). Bioequivalence requirements for generic products. Pharmacology & therapeutics, 62(1-2), 41-55.
Leave a Reply