Design an activity to challenge a specific misconception in math or science using one of the frameworks in Module B and one of the mobile technologies discussed? Explain your pedagogical design decisions.
I chose to challenge 2 common science misconceptions in the area of chemistry relating to heat or temperature:
- Boiling is the maximum temperature a substance can reach.
- The bubbles in boiling water contain “air,” “oxygen,” or “gas,” rather than water vapor.
Establishing “presence” in a simulated or virtual environment can allow a student to engage with the activity and make observations that become relevant.
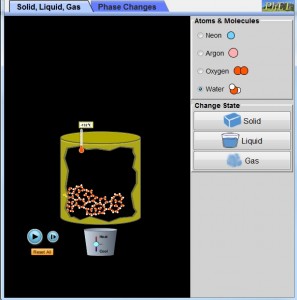
Using the States of Matter simulation developed by PhEt http://phet.colorado.edu/en/simulation/states-of-matter-basics we can immerse students in an experiment that can reveal abstract conceptual theories as an observable simulated visualization.
In addressing these misconceptions I felt the best way to structure an activity was in three steps inspired by SKI framework (scaffolded knowledge integration) explored in Module B. First, provide students with a thought-provoking question. Second, guide students through an inquiry based activity. Third, re-investigate the misconception presented in the question and test for new understanding.
My reasons for using an inquiry process come from a radical constructivist view of transforming traditional pedagogy. The more traditional pedagogy in which Freire (2000) calls the “banking model” because it treats the student as an empty vessel to be filled with knowledge, like a piggy bank. Freire argues for pedagogy to treat the learner as a co-creator of knowledge. The process of engaging students in inquiry based activities using SKI can facilitate students becoming self directed in creating their own knowledge.
Step 1: Thought-Provoking Questions
Students are to be given a scenario addressing 2 common misconceptions:
- Boiling is the maximum temperature a substance can reach.
- The bubbles in boiling water contain “air,” “oxygen,” or “some gas,” rather than water vapor.
- Question 1: “Many people think that once a liquid begins to boil that the substance can not get any hotter in temperature. Do you think this is true?”
- Question 2: “Some people think that once water starts to boil the ‘bubbles’ that come up are made of air or oxygen. What do you think the bubbles are made of?”
Step 2: Inquiry Based Activity
Guide students through using the simulated environment by encouraging them to make observations.
- Using H2O ask student to observe the molecular structure of each molecule at each individual state of matter (solid, liquid, gas). Ask them to identify if there is a difference in the molecule structure when H2O is liquid and when it is gas?
- Ask students to experiment and see if the temperature of H2O can rise beyond 100oC?
Step 3: Test for New Understanding
Revisit the misconceptions and ask students to explain what is taking place.
- Question 1: What is the boiling point of water?
- Question 2: Is the boiling point the maximum temperature water can reach?
- Question 3: What happens to water when it boils?
- Question 4: Are the molecules of H2O something new when they boil?
Continue to engage discussions with students to ensure they understand the correct science and that they do not hold the misconceptions as truth.
References:
Freire, P. (2000). Pedagogy of the oppressed (30th anniversary ed.). New York: Continuum.