I chose to use the framework of technology being applied to simulated virtual worlds in which students embed themselves in order to conduct an experiment.
Turkle’s Question (1997) “Are we using computer technology not because it teaches best but because we have lost the political will to fund education adequately?”, brings up an interesting debate.
One example in the readings that supports using technology with the PhET project Circuit Construction Kit (CCK). The CCK simulates the behavior of simple electric circuits and provides an open workspace where students can manipulate resistors, light bulbs, wires, and batteries. In these studies, students who used computer simulations in lieu of real equipment performed better on conceptual questions related to simple circuits, and developed a greater facility at manipulating real components. (Finkelstein & Adams et al., 2005)
It is interesting to find that Technology can now enable both a spending and resource savings for STEM related experiments as well as produce greater conceptual understanding for students.
Simulations do not necessarily promote conceptual learning nor do they ensure facility with real equipment, but rather computer simulations that are properly designed are useful tools for a variety of contexts that can promote student learning (Finkelstein & Adams et al., 2005).
I chose to present a lesson on the particle theory and the states of mater.
Step 1
Introduce Particle Theory
Particle Theory states:
- All matter is made up of tiny particles
- The particles are in constant motion
- There is space between the particles
- Particles of the same matter are the same
- There is attraction between like particles
Step 2:
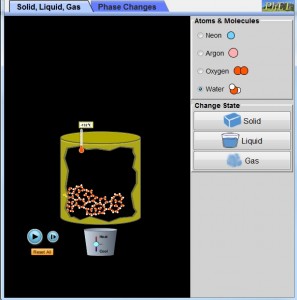
Introduce the Simulated Environment
Using the States of Matter simulation http://phet.colorado.edu/en/simulation/states-of-matter-basics
The learning goals for the simulation will be for students to:
- Recognize that different substances have different properties, including melting, freezing and boiling temperatures.
- Be able to conceptualize what is taking place at the molecular level when substances melt, freeze, or boil.
Similar experiments can be done using ice, thermometers and hotplates however in the virtual environment the student can explicitly observe the concepts behind particle theory and see what is taking place at a molecular level. Simulations do not necessarily promote conceptual learning nor do they ensure facility with real equipment, but rather computer simulations that are properly designed are useful tools for a variety of contexts that can promote student learning (Finkelstein & Adams et al., 2005).
Step 3:
Conduct Simulated Experiment Using Water Molecules
Students should select water and change the state to solid. They will be asked to incrementally increase the temperature to 1 degree Celsius and observe what happens to the molecules. Continue increasing heat to 100oC and observe what happens to the molecules.
Step 4
Connect Observations to Concrete Information
Provide information on the freezing and boiling points of water.
Ask students to answer the following questions:
- Why do they call any temperature below 0 degrees Celsius “below freezing”?
- Joe leaves a pot of water on a stove that is 125oC he came back in 30mis and found that the pot was empty. What happened to the water?
Step 5
Extend knowledge
Allow students to interact with the simulations and make more observations.
- Question students to explain the relationship between temperature of a substance and the speed of particle movement.
- Students should gain the concept that the hotter a substance becomes the faster the particle motion and likewise the cooler the temperature the slower the particle motion.
- The concepts could be further extended toward understanding Kinetic Molecular Theory
Research would suggest that we should provide simulations that are properly designed and applied in the appropriate contexts (Finkelstein & Adams et al., 2005)
References:
Finkelstein, N. D., Adams, W. K., Keller, C. J., Kohl, P. B., Perkins, K. K., Podolefsky, N. S., Reid, S. & Lemaster, R. (2005). When learning about the real world is better done virtually: A study of substituting computer simulations for laboratory equipment. Phys. Rev. ST Phys. Educ. Res., 1 p. 010103. Retrieved from: http://link.aps.org/doi/10.1103/PhysRevSTPER.1.010103 [Accessed: 1 Apr 2014].
Turkle, S. (1997). Seeing Through Computers. The American Prospect, 8(31).