Category Archives: The Projection Section
Annotated Bibliography-FINAL
First Draft
Triggering event for cell dedifferentiation in Physcomitrella patens
Layman’s summary:
Stem cell research is important to many people because of its applications in medicine and disease therapy. However, understanding how to use stem cells in medical treatments is just one side of stem cell research. Another equally important facet to study is how stem cells are maintained and controlled in other living systems. Bryophytes—or mosses—are a unique group of plants that are able to turn any cell in their bodies into an entirely new plant. This ability is not found in any other organism, and how they are able to do this is not entirely known yet. Thus, in this study, I plan to investigate what events trigger cell dedifferentiation that leads to the regrowth of entire organisms from single leaf cells. Specifically, I will be testing whether cells innately contain molecules that prevent each other from degenerating back into stem cells. I will do this by removing cell contents from in-tact leaf cells and observing whether I can induce new plants to grow from the remaining leaf cells. Obtaining a holistic view of stem cell control and maintenance is vital in stem cell research, and it is my hope that understanding the stem-cell like qualities of bryophytes will lead to better understanding of stem cells in human models as well.
Introduction
Cell dedifferentiation is an event that is extremely rare in nature. Although some organisms are able to regenerate—for example, geckos can regrow tails (Alibardi 2009)—the ability to revert cells of a determined fate into one with totipotency is extremely uncommon. Plants can occasionally be induced by hormone baths to produce pluripotent ‘calli’ from cambium tissue (Xuand Hunag 2014), and some species can regenerate apical meristems when they are cut off (Xu et al 2006), but all of these examples involve the degeneration of a particular group of cells. That is, they already possess a pre-designated collection of cells able to regenerate structures, whereas most other cells will not have this ability. For instance, geckos cannot regrow tails using skin cells and plants cannot generally regrow roots using leaf cells. Thus, the ability for an organism to strip any cell of its identity and return it to an embryonic state is extremely rare.
Interestingly, bryophytes are one of the few groups of organisms that can fully dedifferentiate any cell in their body to re-grow an entirely new entity. The ability for bryophytes to dedifferentiate and propagate itself via fragmentation is a trait that has been observed for a long time. The leafy gametophyte, rhizoids, and sporophyte have all been reported to dedifferentiate when excised from the parental plant, making it one of the few organisms that are not limited by particular stem cell ‘niches’ during regeneration (Giles 1971, Westerdijk 1907, Von Wettstein 1924, von Maltzahn 1959). Additionally, it seems that bryophytes do not require any exogenous signals to begin dedifferentiation, and that plant fragments will only begin to dedifferentiate when they are removed from the main structure (Giles 1971). Excised fragments will start to show dedifferentiation in certain cells very quickly—as soon as 24h—and cells destined for dedifferentiation will begin to grow and increase the amount of chloroplasts it has. Then, the cell will asymmetrically divide to form a basal cell and apical cell: the latter of which is nearly identical to young chloronema; a pluripotent filament traditionally seen emerging from spores (Giles 1971, Ishikawa et al. 2011, Sakakibara et al 2014). The apical cell will then act as a new chloronema filament and eventually give rise to an entirely new plant.
[For more information about the bryophyte life cycle and accompanying terminology, see posts in “Project” section on blog]
Unfortunately, the processes and mechanisms surrounding dedifferentiation in bryophytes is complicated and fairly inconsistent across species. Some species like Funaria will produce numerous apical chloronema cells across entire excised leaves, whereas species like Physcomitrella patens will produce protonema from just cells bordering the detached boundary of the leaf (Giles 1971, Westerdijk 1907). Others yet do not differentiate at all: the genus Dawsonia will increase chloroplast number and cell size but ultimately never form functional protonema (Von Wettstein 1924).
Not all cells are created equal either. Often, there is a gradient of most differentiable tissue to least, such that sporophytic tissue will dedifferentiate more readily near the apex whereas protonema will dedifferentiate better near the basal area than the tip (Von Wettstein 1924). Furthermore, the fact that sporophytic tissue and gametophytic tissue (which are diploid and haploid, respectively) are able to give rise to the same kind of chloronema is rather strange. This may be due to the fact that all gametophytic tissue in bryophytes are arrested in the G2 phase of the cell cycle, which means they all possess a duplicated (but still haploid) genome (Ishikawa et al 2011). This is in contrast to all other land plants and animals, whose cells are usually stopped in the G1 phase (Ishikawa et al 2011). Indeed, the dedifferentiation process of bryophytes is complex and seemingly difficult to understand.
There have been a handful of papers investigating the cellular changes associated with dedifferentiation in mosses. Ishikawa et al (2011) is a particularly notable example. Not only were they able to establish the duplicated state of the gametophytic genome, but also identified two essential proteins involved in triggering the dedifferentiation event in P. patens: CDKA and CDKD (cyclin-dependent kinases). It appeared that CDKA was an activator for CDKD, and while CDKA was found to be present in all cells at all times, CDKD was only transcribed in cells at detachment sites. Sakakibara et al (2014) has also contributed to the knowledge about bryophyte dedifferentiation by identifying a homolog of stem cell regulators in flowering plants (PpWOX13L) that is essential for the initiation of growth prior to the asymmetrical division of pre-chloronema. However, although both of these studies offer insight into what pathways are active post-trigger, the actual triggering mechanism for dedifferentiation in mosses still remains a mystery.
There have been some theories as to how cells are able to trigger dedifferentiation in excised plant fragments. Von Maltzahn (1959) suggested that leaves (and other tissue) receive signals form the apex that specify not to dedifferentiate, and that when leaves are removed from the main plant, the lack of this signal may result in spontaneous activation of CDKA/CDKD or PpWOX13L pathways. This idea of an innate ‘instability’ in bryophyte systems can be supported by similar systems seen in angiosperms. Higher plants are able to react to injury by using auxin as a concentration-dependent signal. Normally, there is a constant auxin flow from the apical tip to the peripheral tissues, but when tissues are damaged along this flow it slows or blocks the flow of auxin. This results in a build up of auxin on the basal side and a deficiency of auxin on the apical side, which initiates transcription to begin healing and regeneration (Asahina et al 2011, Read and Ross 2011). One can imagine how bryophytes may use a similar system to signal to leaves when dedifferentiation is necessary.
Other possible triggers for dedifferentiation include the use of external cues or hormones. The protonemata in bryophytes use hormones to communicate or detect each other by excreting substances into the environment. It is then the concentration of these hormones that permit or restrict bud formation. It is possible that excised leaves may be triggered to dedifferentiate because of similar reasons; namely because of the build-up of excreted residues that self-promote dedifferentiation when in contact with soil or media. In addition, previous studies have shown that light can have an inductive effect on chloronema (Giles 1967, Maltzahn 1968) so it would not be out of the question for light to play a role in dedifferentiation activation.
Finally, it is also possible that wounding may directly initiate the transcription process for cell dedifferentiation by triggering the transcription for wounding factors. This would be in contrast to the polarity theory, in which there is an absence of factors rather than the addition of them. Perhaps exposure of cell cytoplasm to differential osmotic conditions causes differential gene expression, leading to cell dedifferentiation. This model would explain why some plants might show ubiquitous cell dedifferentiation and some only show it on cells bordering cut sites: The leaves removed from certain species may be more delicate than others, and are thus more prone to damage than others.
Thus, despite our increasing knowledge of bryophyte dedifferentiation, we have yet to determine how the process is actually triggered: a question that I plan to address.
I hypothesize that there is the presence of some ‘constant’ signal in each cell that stabilizes neighbouring cells. Consequently, I propose that by removing the cytoplasm of neighbouring cells, I can induce cell dedifferentiation due to the removal of these ‘stabilizing’ signals.
Significance
Stem cell research is important for a variety of reasons. In medicine, there is hope that stem cell therapy can cure diseases or regrow structures to help both humans and animals. Thus, the side of stem cell research the general public hears about predominantly revolves around direct human application. However, there are many avenues of study that surround stem cell research that are equally important.
It is important to understand how stem cells function and how they stay totipotent because that information might help us understand how to make treatment more effective. Additionally, learning about how other systems handle stem cells may lead to analogous pathways within our own bodies. Other applications of cell totipotency can be used in farming techniques. Being able to master cell dedifferentiation to create cloned strains of certain foods may be beneficial to many farmers and save time and money. Clearly, there are many reasons why we would want to learn more about stem cells. Bryophytes give a unique perspective into stem cell maintenance because they are able to dedifferentiate every single cell in their body to become totipotent. Thus, understanding the triggering events and pathways involved in cell dedifferentiation may lead to better understanding of how to reverse or halt cell differentiation in other systems. In summary, the pieces of information gained from this experiment can eventually be applied to the direct problems that humans face.
Experimental Approach:
For my experiment, I plan to remove the cytoplasm of leaf cells from in-tact gametophytes. I chose to use the species Physcomitrella patens because of its historical use in bryophyte research. It is commonly used in gene studies because homologus recombination in this system is fairly simple, and reproducing clones is easy due to its ability to regenerate by fragmentation. Additionally, since Physcomitrella patens is part of the class Bryophyta, it generally does not possess any ‘special’ features associated with some other classes of bryophytes. Class Bryophyta is often referred to as the “Have-nots” of the bryophyte world because they are used as the ‘null’ comparison for other traits specific to other classes. Thus, P. patens can provide a simple, effective model to broadly represent bryophytic characteristics.
The use of the reporter genes used in my experimental was inspired by the system created by Shaefer (1994) and Ishikawa et al (2014). All protocols have been successfully used in other experiments.
Culturing of P. patens
First, I will obtain a line of P. patens similar to the ones found in Ishikawa et al. (2011). Preferably, I would like to use the ProCYCD;1:NLS-GFP- GUS #263 P. patens line in their paper, which used the polyethylene glycol–mediated transformation system created by Nishiyama et al (2000) and Shaefer (1994) to introduce a GFP signal to the CDKD protein. If obtaining this strain is not possible, then I will simply re-create this strain from WT P. patens using the protocols found in the Ishikawa et al (2011) and Nishiyama et al (2000) papers. Cultures will be kept on BCDAT media, which is used for cultivation of protonema and gametophores (Ishikawa 2011, Hiwatashi and Hasebe 2004). I have attached a copy of the BCDAT recipe in the supplemental information.
Ten stock plates of P. patens will be grown in case of error. They will be incubated at 250 C in continuous white light using BCDAT media (Nishiyama et al 2000) and transplanted every 8 weeks to ensure optimal health. When growing experimental gametophytes, protonema will be isolated from stock plates and rinsed with sterile BCDAT liquid media three times before placing on a layer of autoclaved cellophane, which will then be placed on solid BCDAT media. Rinsing with liquid media will minimize contamination from bacteria and fungus, whereas the cellophane layer will prevent new protonema from growing into the media and consequently make isolation of plants easier. Experimental plates will be cultivated for 4 weeks at 250 C to produce gametophytic shoots (Ishikawa et al 2011), which will then be removed with tweezers, rinsed 3 times in 0.22um filter-sterilized water, and placed horizontally on cellophane on BCDAT plates.
Treatments
All treatments will begin with a plate of freshly inoculated P. patens as described above. There will be 14 plates total with a single gametophyte on each. Ten of these gametophytes will be subject to all four treatments on different leaves to account for differences in individuals or media. The remaining four gametophytes will have 3 replicates of each type of treatment to ensure treatments are not affecting the results of each other. Treatments will be randomized in position along a single gametophyte so as to prevent any leaf-age effects. Leaves for treatment will be chosen based on how flat against the media they are: leaves that lie flat on the cellophane will be preferentially chosen to ensure any budding protonema will have sufficient nutrients.
Cytoplasm will be removed by glass micropipettes stretched out to have a tip with a 0.2um diameter (Mackler 1992). Originally, the extraction of cell contents was done on hippocampal neuron cells but as far as I am aware, such procedures have also been done on plant cells without issue. The cells of bryophytes are very delicate so the integrity of the cell wall should not pose a problem.
- No-cytoplasm treatment
Using the micropipette, an entire row of cells across the broadest part of the leaf will have their cytoplasm removed. The removal of the cytoplasm will be done by aspirating the contents out under observation via a 10X dissecting scope and visual examination of the cells will confirm the absence of cytoplasmic contents. This will be done for 10 leaves on 10 different plants.
- Puncture-control (Negative control 1)
Using the same micropipettes and procedures as in treatment (1), cells will be punctured but not aspirated.
- Excise-control (Positive control)
Using a razor blade, single leaves will be cut at the broadest part and moved 1cm away from the main shoot using sterilized tweezers. We will be careful not to damage the cells as we are moving the leaf fragment.
- In-tact control (Negative control 2)
Leaves will remain in-tact and un-punctured on the main stem.
Observation
Using a light microscope, I will make and record observations at 1h, 12h, 24h, 48h, and 2 days. The following traits will be noted:
- chloroplast count (with counts from surrounding cells for comparison)
- Budding or abnormal growth (made between time points)
- Cell division (the formation of the apical chloronemal cell)
Additionally, I will also look at GFP fluorescence under UV light to evaluate CDKD expression.
Finally, I will look at total protonemal growth after 2 days.
Possible Results:
No-cytoplasm treatments:
If my hypothesis is correct, I expect to see cells growing into protonema on the ‘No-cytoplasm’ treatments. The cells bordering the row of no-cytoplasm cells are expected to be the ones giving rise to chloronema. If cells on both sides grow chloronema, it would suggest that each cell has the same stabilizing signal, and that there is no polarity regarding this signal. However, if there were some kind of polarity (for example, if the signal is being excreted by the apical growth cell), then one would expect only the cells apical of the no-cytoplasm divide to dedifferentiate into chloronema. Another possible result would be for the no-cytoplasm treatments to lack dedifferentiation in any cell at all. These results would suggest that there is no ‘stabilizing’ signal, but that dedifferentiation is likely triggered by differential gene expression in response to cell damage. With no cytoplasm (and thus no transcriptional machinery), there would be no chance for cells to express wounding signals. If this result occurred, it would have to be compared to the puncture-controls to see if injury alone can initiate cell dedifferentiation.
In terms of CDKD-GFP expression, I would expect the cells along the line of no-cytoplasm cells to show high expression at 24h and 48h (Ishikawa et al 2011), and for this expression to coincide with chloronema formation. An unexpected result would be if CDKD-GFP expression and chloronema formation were not displayed together: this would suggest that either CDKD activation is not exclusive to dedifferentiation, or that it may not be necessary for dedifferentiation.
Puncture-control treatments:
Assuming the puncture-control cells did not lose too much cytoplasm and that they retained the ability to repair themselves, I would expect to see no chloronemal growth. However, it is possible that the puncture would be too large and result in enough loss of cytoplasm to initiate cell dedifferentiation on neighbouring cells. I would attempt to observe any gradient-like changes that occur in the no-cytoplasm, puncture control, and complete excision treatments to see if the puncture control would have less response than the no-cyotplasm and complete excision controls. Additionally, I would use visual examination to observe how damaging the puncture is. If it were evident that the puncture control had lost a substantial amount of cytoplasm, then I would have to find a new way to remove the cytoplasm while minimizing cell damage.
The expected result would be to see CDKD-GFP expression in the cells neighbouring the punctured cells. If this were the case, you would also expect protonemal growth from the neighbouring cells. An interesting result would be if CDKD-GFP expression existed in the punctured cells more than the neighbouring cells. It would suggest that it is physical damage that directly initiates cell dedifferentiation. This would be compared with possible protonemal growth to see if it corresponds with the increase in CDKD-GFP expression. CDKD-GFP expression in the punctured cells would also suggest that removing the cytoplasm of damaged cells would prevent dedifferentiation in leaves as a whole. This is because CDKD-GFP expression in punctured cells would mean differential gene expression in only damaged cells, and that dedifferentiation is not due to the loss of neighbouring ‘suppressors’ or some kind of polarity signal. Thus, one would expect that if the punctured cells showed CDKD-GFP expression, then the no-cytoplasm cells would not show dedifferentiation at all.
Thus, if CDKD-GFP expression were found in the punctured cells, one would expect no CDKD-GFP expression or protonemal formation in the no-cytoplasm treatment because there would be no damage done to the neighbouring cells. This is in contrast to the possibility where no CDKD-GFP expression would be seen in the punctured cells but high expression in the neighbouring cells, which would support my original hypothesis regarding the presence of a ‘stabilizing’ factor.
Excise-control treatments
I am expecting the excise-control to show protonemal growth from the cut boundary and CDKD expression in those cells as well. This treatment has already been tested successfully by Ishikawa et al (2011) under nearly identical conditions; so any deviations from this result would suggest we have done something wrong. The only difference between Ishikawa et al (2011)’s treatment and my experiment is the presence of the whole-gametophyte 1cm away from the excised leaf. In the Ishikawa et al (2011) experiment, they transferred the excised leaf onto a different plate. Thus, if our results differ from theirs, it would suggest there is some signal from the main shoot that plays a part in controlling dedifferentiation. This signal could be cytoplasmic (which may diffuse across the agar) or exogenous (such as the hormones found excreted by protonema). Alternatively, there may be multiple pathways in activating cell dedifferentiation and there would need to be more investigation as to why certain pathways are activated at certain times.
In-tact treatments
It is expected that these leaves will not show any dedifferentiation because they were not wounded in any way. Most literature has stated that attached leaves will not grow protonema, but there have been a few observations of this happening. It is unclear why this is the case, but I suspect it might be because of damage done to the leaves when they are placed on agar during the whole-gametophyte transferring process. Another possibility would be that there may be hormonal signals constantly excreted by the main shoot that signal dedifferentiation, and that when the plant is erect, these signals naturally dissipate into the air or are washed away by rain. However, when plants are placed horizontally and are in contact with solid substrate, these signals accumulate around the leaves and signal dedifferentiation.
If the scenario described above occurs, I would suspect that there are multiple pathways to dedifferentiation because the exogenous hormone accumulation theory would not explain why only cells bordering excised leaves would sprout protonema. If the leaf were excreting a hormone that accumulates around itself to induce dedifferentiation, one would expect the centre leaf cells to be most prone to dedifferentiation because it would have the highest concentration of these accumulated hormones.
Supplemental- BCDAT media
INFORMATION FROM GOOGLE SEARCH– I DID NOT WRITE THIS
- Cultivation
2.2 Cultivation of protonemata and gametophores (originally written by Yuji Hiwatashi, and edited by Mitsuyasu Hasebe: last updated 16 June, 2004)
BCDATG medium is used for regular cultivation. Protonemata grow faster in BCDATG medium than in other media, and is good to collect materials for nucleic acid extraction in relatively short period. However, senescence of plants is a bit enhanced in BCDATG, and BCDATG should not be used for long culture. Another good point of BCDATG is easiness to detect contamination because of glucose in the medium.
BCDAT medium is mostly similar to BCDAT, but growth of protonemata is a bit slower. We use BCDAT for selection with G418 and/or hygromycin after transformation, because of its long selection period. Hard to detect contamination (contaminants also grow slower), and should be careful.
Protonemata grow slower in BCD + 1 mM Ca2+ medium (often called just BCD medium), but differentiation of chloronemata and caulonemata is more easily observed in BCD medium than other media. Furthermore, irregular cell divisions at young stages of bud development observed in other media are not observed in BCD medium, and should be good for bud observation.
2.2.1 stock solution
store at 4C. Stock solution D is easily oxidized, and should be used in 2 to 3 months.
stock solution A(×100)
| Ca(NO3)2・4H2O | 118 g (0.5 M) |
| FeSO4・7H2O | 1.25 g (4.5 mM) |
| up to 1000 ml with H2O |
stock solution B(×100)
| MgSO4・7H2O | 25 g (0.1 mM) |
| up to 1000 ml with H2O |
stock solution C(×100)
| KH2PO4 | 25 g (1.84 mM) |
| adjust pH to 6.5 with 4M KOH | |
| up to 1000 ml with H2O |
stock solution D(×100)
| KNO3 | 101 g (1 M) |
| FeSO4・7H2O | 1.25 g (4.5 mM) |
| up to 1000 ml with H2O |
Alternative TES(×1000)
| CuSO4・5H2O | 55 mg (0.22 mM) |
| H3BO3 | 614 mg (10 mM) |
| CoCl2・6H2O | 55 mg (0.23 mM) |
| Na2MoO4・2H2O | 25 mg (0.1 mM) |
| ZnSO4・7H2O | 55 mg (0.19 mM) |
| MnCl2・4H2O | 389 mg (2 mM) |
| KI | 28 mg (0.17 mM) |
| up to 1000 ml with H2O |
500mM Ammonium Tartrate(×100)
| Ammonium Tartrate | 92.05 g |
| up to 1000 ml with H2O |
50mM CaCl2(×50)
| CaCl2・2H2O | 7.35 g |
| up to 1000 ml with H2O |
→autoclave
2.2.2 regular media
BCD + 1 mM Ca medium (BCD medium): 1000 ml
| H2O | 900 ml |
| stock solution B | 10 ml |
| stock solution C | 10 ml |
| stock solution D | 10 ml |
| Alternative TES | 1 ml |
| 50mM CaCl2・2H2O(if use powder) | 20 ml(=1 mM)(0.15 g) |
| agar(Sigma A6924) | 8 g(=0.8%) |
| up to 1000 ml with H2O |
→autoclave, pour to 90 mm petri dishes. After agar was solidified, open a lid, keep drying for 30 min at r.t. in cleanbench. These plates are preserved at r.t.
BCDAT medium: BCD + 1 mM Ca + 5 mM ammonium tartrate medium: 1000 ml
| H2O | 900 ml |
| stock solution B | 10 ml |
| stock solution C | 10 ml |
| stock solution D | 10 ml |
| Alternative TES | 1 ml |
| 500mM ammonium tartrate. | 10 ml(final conc. =5 mM) |
| 50mM CaCl2・2H2O(as powder) | 20 ml(final conc. =1 mM)(0.15 g) |
| agar(Sigma A6924) | 8 g(final conc. =0.8%) |
| up to 1000 ml with H2O |
BCDATG medium: BCD + 1 mM Ca + 5 mM ammonium tartrate + 0.5% glucose medium: 1000 ml
Add 5 g glucose to 1000 ml BCD medium
2.2.3 special reagents
Thiamine chloride (M.W. 337.3)
0.5 mg par 1000 ml medium (final conc. 1.5 uM)
p-amino benzene (M.W. 137.1)
247 ug par 1000 ml medium (final conc. 1.8 uM)
2.2.4 cultivation conditions
Light:
We usually use continuous light (40 umol photons m-2s-1)conditions or 16 light and 8 hours dark conditions. When you prefer to induce gametangia, short day conditions are better (see 2.3).
Temperature:
25C for regular cultivation.
15C for gametangia induction.
2.2.5 vegetative propagation of protonemata
You can preserve protonemata at 4C under dark or low light conditions for long period. To prevent drying up, seal a petri dish with parafilm.
To vegetative propagation of protonemata, use young protonemata cultivated for 5 to 7 days.
Preparation
- BCDAT or BCDATG medium
- distilled water
- cellophane
pretreatment of cellophane
Some cellophane prohibits Physco growth. You should compare the growth with or without cellophane.
- Cut cellophanes to a little bit smaller than the size of the plate (the same one as used for E. coli culture). A circle cutter is useful to cut cellophan.
- Dip in 5 mM EDTA (pH 8.0) in a glass plate, then autoclave (120C 20 min). You can put more than 30 sheets of cellophanes in a plate.
- After autoclaving, wash with MilliQ water for several times to remove EDTA.
- Dip cellophane in MilliQ water, then autoclave (120C 20 min).
- These cellophane seats are used to overlay on agar media and prevent protonemata from growing into the media.
- Polytron: we usually use model PT2100 or MODEL K with DA2121/2 shaft. You can use motor and pestle instead of polytron.
Procedures (all procedures should be done in cleanbench)
- put a seat of cellophane on each medium.
- Pour 20 ml distilled water in a glass test tube.
- Remove protonemata from culture dish using forceps, and move to the glass test tube.
- Digest with “polytron PT2100” at mimimu power (in case of “MODEL K power 4 to 5) for 10 sec.
- Pour 2 ml digested green solution on a cellophane-covered plate with pipet.
- Cultivate at 25C.
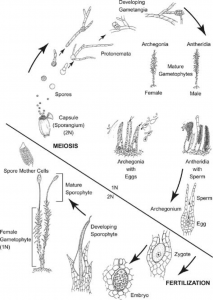
Supplemental- Bryophyte Lifecycle
Supplemental Information
Bryophyte Lifecycle
- Note: the protonemal stage is divided into two parts: chloronema and caulonema. Chloronema grows first and is characterized by bright green, round chloroplasts. Caulonema eventually develops and is faster growing than chloronema, with darker, spindle-shaped chloroplasts.
First Outline (26March2015)
Outline of Final Project
INTRO:
Regeneration is regrowth of structures from a collection of stem cells
- Regeneration occurs in both animals and plants
- Limbs of geckos (Alibardi 2009)
- Crassulaceae and Cactaceae families can regrow roots from detached leaves on soil (Xu & Huang 2014)
- Dedifferentiation is rare—and much more common in plants
- Can cut off apical tips that harbour pluripotent cells and the plant will de-differentiate ends to make new pluripotent cells (Xu et al. 2006)
- But interconversions generally happen between roots-roots or shoots-shoots. There are some limitaitons
Most organisms do not have de novo dedifferentiation though
- plants can be induced to dedifferentiate, but it requires the intervention of plants hormones (Xu & Huang 2014)
- high auxin, low cytokininà forms callous from which new shoots can grow
- dedifferentiation is not complete—need certain ‘collection’ of regenerative cells
- procambium and cambium can give rise to new roots and shoots, but other cells can’t
- Bryophytes, on the other hand, CAN dedifferentiate.
Quick recap of bryophyte lifecycle:
- Begins as chloronema. Uniseriate, photosynthetic, round chloroplasts (Vanderpooten and Goffinet 2010, Kofuji 2014)
- Then, will grow into fast-spreading caulonema (Kofuji 2014)
- Caulonema and chloronema and collectively known as protonema
- Form buds, which form gametophyte (1N) stage
- Gametophyte is main stage of life—it produces gametes (sperm and egg) and forms a sporophyte (2N)
- Short sporophyte phase will produce spores, which are spread and germinate into new protonema
Bryophytes can dedifferentiate from all states—a trait observed a long time ago.
- leafy gametophyte, rhizoids, and sporophyte all do this (Giles 1971, Westerdijk 1907, Von Wettstein 1924, von Maltzahn 1959)
- if they stay attached, they will not dedifferentiate (Mnium affine) (Giles 1971).
- Once cut, they will show changes in certain cells
- Chloroplasts grow and divide (Giles 1971, Ishikawa et al 2011, Sakakibara et al 2014)
- Divide asymmetrically; top cell becomes new protonema (Ishikawa et al 2001,
- Grows a whole new plant
BUT it gets complicated.
- some species will grow tonnes of protonema (Funaria); some will grow protonema JUST from detached boundary (Physcomitrella patens); and others won’t dedifferentiated at all (Dawsonia) (Giles 1971, Westerdijk 107; Von Wettstein 1924)
- not all cells are equal either; there is often polarity associated with dedifferentiatbility
- sporophytes dedifferentiate easier near the apex (Von Wettstein 1924)
- protonema will dedifferentiate better at basal area than tip
- Cells are arrested in G2—which mean they have a duplicated haploid genome! (Ishikawa 2011)
Possible mechanisms for triggering dedifferentiation:
- there is overall instability in system—so when you tear leaf off, you throw off the polarity of cells
- von Maltzahn (1959) suggested leaves get signals from apex to tell them not to dedifferentiate
- plants have similar signal for wounding: they have a constant auxin flow and when there is a wound, auxin builds up on the basal side and lacks on the apical side, so it changes transcription to initiate healing (asahina et al 2011, read and ross 2011)
- thus, likely due to expression of thousands of different genes (Xiao et al 2012)
Other possible triggers:
- external cues and hormones
- regular protonmea communicate to eachother via Factor H and Factor F— it would not be impossible for similar things to happen in gametophyte
- light and dark appears to sometimes affect which way the plant will dedifferentiate, so perhaps you need external cues too?
Finally, it’s possible that the trigger is due to the direct production of wounding-proteins
- this is in contrast to the ‘lack’ of polarity theory, which involves the ABSENCE of certain factors
- this might make sense because there have also been reports of mosses that redifferentiate while still attached to main plantà perhaps the leaves got damaged
- or, the species that show lots of dedifferentiation maybe got ‘damaged’ more and thus shows more protonemal growth
Goal is to determine the TRIGGER for dedifferentiation in physcomitrella patens
- two main papers are about the pathways causing cell dedifferentiation
- Ishikawa et al (2011) discovers that CDKA is always present in cells (polar argument evidence?) but something triggers it to activate CDKD when leaf cells are cutà results in dedifferentiation
- Sakakibara et al (2014) found a homeobox 13-like gene (related to stem cell regulators in flowering plants) that increases when you cut cells
- My hypothesis is that there is some sort of ‘constant’ signal throughout the plant that makes it stable, and removing cytoplasm will cause dedifferentiation
- Want to remove cytoplasm, but keep leaf cells in-tact
Studying stem cell regulation has always been of interest to us
- new therapeutic methods in medicine
- learn about development and important regulators that may cause genetic defects
- learn how to improve crop yields or produce clones of desirable plants
Studying in moss is interesting because it is different!
- shows more plasticity than ANY other plant
- we want to know WHY and HOW
- perhaps we can apply moss abilities to other organisms and improve culturing techniques, medical treatments, and general knowledge
Experimental approach:
- Obtain strain of Physcomitrella patens similar to one in Ishikawa et al (2011) paper
- If possible, obtain the one with the CDKD-GFP-GUS transformation
- If not, follow the procedures in Nishiyama (2000) and Shaefer (1994) to produce a strain of Physcomitrella patens with a GFP reporter enzyme attached to the CDKD protein via homologous recombination.
- Raise several back-up strains—grow on BCDATG media for regular growth, and BCDAT for maintenance growth. The latter just makes protonema grow slower, which would make it easier to take care of.
- Pour petri plates of media covered in autoclaved cellophane; germinate with paten on top of cellophane to prevent protonema from growing into agar: makes it easy to remove whole-gametophytes and protonema
- Treatments: For all treatments (except positive control), I will remove WHOLE gametophytes (4 weeks, Nishiyama 2000) and place horizontally on new media with cellophane. I will gently ensure some leaves are pressed down on the media. These leaves will be used for experimentation. Each gametophyte will have one of each type of treatment to ensure control of variables across treatments. Each treatment will have 5 plate replicates with 3 treatment leaves on each plate. This means each treatment will have a total of 15 leaves treated. After all treatments, plates will be incubated at 25 degrees C with constant light (Shaefer 1994).
- REMOVAL OF CYTOPLASM (Treatment)
- Use glass micropipette (stretched out vertically to produce small pore of 0.2um, as described in Mackler 1992)
- Mackler removed cytoplasm from neurons, but I will do it in bryophytes
- Remove cytoplasm from cells across the centre of the leaf cell; go all the way across the cell
- Use glass micropipette (stretched out vertically to produce small pore of 0.2um, as described in Mackler 1992)
- REMOVAL OF CYTOPLASM (Treatment)
- Incubate
- PIPETTE CONTROL
- To control for pipette prick, repeat procedure for 1(a) except do not remove cytoplasm.
- Incubate
- NEGATIVE CONTROL
- Do not damage or remove leaves from plant
- Incubate
- POSITIVE WOUND CONTROL
- Using sharp knife, cut a straight line across a leaf, making sure to damage the entire line of cells
- Keep leaf tip ‘attached’ to leaf base
- POSITIVE DETACHMENT CONTROL
- Using sharp knife, cut a straight line across a leaf
- Remove leaf tip and relocate 1cm away from rest of plant
- Using a light microscope, observe plants at 1h, 6h, 12h, 48h, and 2 days and mark/observe the following traits:
- Chloroplast count
- Budding or abnormal growth
- Cell division
- At the 2 days mark, protonemal growth should have appeared already (Ishikawa et al 2011)
- I will compare the above characteristics with CDKD-GFP expression under UV light at each time point. I will also try to characterize specific light intensities
- Compare relative success of dedifferentiation across all treatments.
Possible results:
If my hypothesis is correct, then I expect that by removing cytoplasm
Annotated Bibliography draft (From 14March2015)
Xu, L., & Huang, H. (2014). Genetic and epigenetic controls of plant regeneration. Current topics in developmental biology. 108: 1-
- tissue/organ repair AND generation of new plants (Birnbaum & sanches Alvarado, 2008); sgimoto, Gordon & meyerowitz, 2011).
- Tissue colture, you can see when adding hormones (sussex 2008, gautheret 1983, Thorpe 2006, 2007)
- Animals can regenerate—but this requies movement of stem cells; plant cells can’t move
- De novo organogenesis—grows from cut pieces
- Arabidopsis thaliana—need callus-inducing medium with high auxin and low cytokinin.
- Somatic embryogenesis—single somatic cell turns into embryo of plant.
- Yang and zhang 2010
- Suggests totipotency of cells; verdeil, alemanno, niemenak, tranbarger 2007)
- Xu et al 2006à can regenerate root tips by cutting of cells; auxin accumulates in new ‘end’ cells and causes protein cascade which transforms QC-adjacent cells into new QC cells
- Feldman 1976—showed that root tips can be quickly regenerated into a new QC (quiescent center)[ at tip of root apical meristem (RAM) and has surrounding stem cels; hs 2-4 cells that divide very slowly; controlled by TF WOX5. (Sarkar et al 2007)
- sena et al 2009à showed stem cell niche was not needed; it cut off QC instead of lasering it off. (abalation)
- also high levels of auxin involved
- using auxin polar transport-inhibitor NPA (naphtylphthalamic acid)à blocked QC regeneration
- must be within 130um; higher means less ability to regenerate
- WOUNDING
- Basipetal auxin stream blocked at wounded positions (asahina et al 2011, reid and ross 2011)à high auxin, low auzin below. Activates high and low promoters and both needed for healing
- Also need jasmonic acid and ethylene (asahina et al 2011)
- DENOVO ORGANOGENESIS
- We need to induce via calluses; but in nature perhaps you produce less obvious callus
- Callus formation is not actualy an undifferentiated totipotent group of cells; actually, it is a mass of root meristem tip cells that resembles lateral root formation (Atta et al 2009, che, lall, howell 2007, he, chen, huang and xu, 2012; sugimoto, jiao, meyerowitz 2010)
- Callus formation initiated with divisons from xylem-pole pericycle cells (atta, che), which is also where lateral roots come from (benkova and bielach 2010; peret et al 2009)
- Callus no longer totipotent; is pluripotent bc not dedifferentiated; its transdifferentiated (sugimoto et al 2010, 2011)
- PcG (polycomb group) in mutants do not allow de-regulation of ‘leaf’ genes—so they thing PcG plays a role in supressing leaf genes. Done by depositing H4K27me3 histones on leaf gene loci (he et al 2012)
- De novo root organogenesis—in Arabidopsis, can see new roots originate form vascular procambium or cambium (ahkami et al 2009; correa lda et al 2012; greenwood, cui, Xu 2001)
- – suggests that auxin needs to be involved; because blocking it prevents rooting process.
- Possible similar to lateral formation form hyopcotyls
- Wound signal—absent during lateral or adventitious root formation from hypocotyls; wound signal not yet defined.
- Xylem-pole pericycle, preprocambium, procambium, cambium – all have different stem cell features
- Procambium and cambium= pluripotentn vascular peristem in primary and secondary development of vascular tissues, respectively (elo, immanene, nieminen, helariutta 2009)
- Preprocambium is progenitor of procambium (sam as above)
- Xylem-pole pericycle cells give rise to cambium *rost, barbour, stocking and murphy 1997)
- Seems like you NEED stem cells to regenerate roots/shoots
- CARROT SOMATIC EMBRYOGENESIS
- Steward, mapbs, mears 1958
- Very complex; involves hormones, TF, epigenetic reg, yang and zhang 2010)
- Somatic embryogenesis
- Balance between GA and abscisic acid (ABA)—(de castro and hilhorst, 2006; hays, mandel, pharis 2001); hu et al 2008; ogawa et al 2003; Phillips et al 1997; etc etc…
- Embryo cells: low ratio of GA to ABA; ratio higher in somatic cells (braybrook and harada 2008)—suggested that auzin might induce/initiate somatic embryogenesis but changes in GA/ABA ratio may provide suitable environment for cells to become comptent. Also, ethylene may be involved. (bai et al 2013, piyatrakul et al 2012; zheng, zheng, perry 2013)
- Two classes of TF important in Arabidopsis: LEAFY cotyledon (LEC) genes and agamous-like15 (AGL15)—both only expressed in embryo and overexpression shows embryo-like symptoms
- AGL15 might inhibit GA pathway and upregulated auxin signalling gene (Zheng et al 2009)
- LEC2 promotes auxin pathway—all these make up genetic network that promotes ABA and auxin, but inhibits GA
- Some interplay between pathways; regulate each other
- Epigenetic regulation of somatic embryogenesis
- pcG and PICKLE (PKL)pathways)—mutations in both can result in somatic embryogenesis
- PcG retains somatic ell identity
- Arabidopsis: PRC1 and PRC2 are PcG complexes.
- PRC2—mediates H2K27me3 leaf-to-callus formation (he 2012) and also repressing embryonic genes during embryo-to-seedling phase (bouyer et al 2011)
- PCR2= incomplete transition from embryo to seedling; mutant seedinly has disorganized cell divisions (bouyer et al 2011)
- Lec1, lec2, fus3 extopicaly expressed in PRC2 mutant (Makarevich et al 2006)
- Genome-wide analysis of H2K27me3 suggests embryo-sepcific genes are highly trimethylated in somatic cells (Zhang, Clarenz eta l 2007)
- PCR1: contain 4 major proteins: LHP1 (like heterochromatin protein1, atRING1a/b, AtBMI1a/b and EMF1
- LHP1 recognizeds H3K27me3; second two are responsible for H2A ubiquitination after PRC1 bins to targets (Bratzel et al 2010);
- Mutations in a/aà Lecs, AGL15, etc (Bratzel et al 2010 chen et al 2010)
- PRC1 and PRC2à may function together to control the same embryonic genes
- Epigenetic PKLà codes CHD-type ATP-dependent chromatin remodelling factor; LOF first identified as GA-deficient mutant (Ogas, cheng, sung and Somerville 1997; 1999)
- Mutations in PKL- ectopic expression of LEC1/LEC2, FUS3
- WOUND SINGAL
- All three types of plant regeneration triggered by wounding
- Changes hormone biologyà gene expression
- Molecular nature of wound singal remains unclear
- Possible suspects: plasma transmembrane potential; Ca; reactive oxygen species; palnt hormones; metabolic processes (leon, rojo, sanches-serrano 2001; maffei, mithofer, boland 2007)—complex bc could also trigger defense
- In arapidopsis, wound induced dedifferentiation 1 (WIND1) (iwase et al 2011; iwase, ohme-takagi, sugimoto 2011)à induced at wounded region (but don’t know how) and seems to regulate via cytokinin pathway (same as above)
- MOSS REGENERATION
- Physcomitrella patens—regeneration possible in many tisues and cells and is easily triggered
- Think it might be due to change sin expression of thousands of genes (xiao, zhang, yang, zhu, he 2012)
- Ishikawa—CDKA was induced priper to transition and DNA synthesis inhibitors (aphidicolin) could NOT stop transition and did not block induction of protonema-specific genes.
- Suggests cell-fate transition occurs before and independent of cell division
Sakakibara, K., Reisewitz, P., Aoyama, T., Friedrich, T., Ando, S., Sato, Y., Tamada, Y., Nishiyama, T., Hiwatashi, Y., Kurata, T., Ishikawa, M., Deguchi, H., Rensing, S.A., Werr, W., Murata, T., Hasebe, M., & Laux, T. (2014). WOX13-like genes are required for reprogramming of leaf and protoplast cells into stem cells in the moss Physcomitrella patens. Development. 141: 1660-1670.
- cells at wound margin of detached leaves become reprogrammed into stem cells
- patens WUSCHEL-related homeobox 13-like genes (PpWOX13L)—homologs of stem cell regulators in flowering plants—upregulated and required for initiation of cell growth during stem cell formation
- Deletions fail to upreg genes encoding cell wall hoosening factor homologs
- Mutant sygotes fail to expand and initate an apical stem cell to form the embryo
- Analogous to WOX stem cell functions in seed plants, but using different cellular mechanism
- Dedifferentiation occurs more readily in plants (Birnbaum and sanchez Alvarado 2008)
- NATURAL CONDITIONS? (Steeves and sussex, 1989)
- Prigge and bezanilla 2010* similar to ishikawa?
- Saw ppWOX13LA and ppWOX13LB throughout life cycle, but no ppWOX13LC at any developmental stage
- Used GFP knock-in and saw it in all nuclei examined—but higher in apical cells of chl/caul vs subapical, and higher in egg/zyg compared to surrounding
- Did detached leaf assay—saw whole-leaf ppWOX13LA?B transcript levels transient increase after detachment
- First 12h; all cells increased slightly—afterward, only cells facing cut site and that eventually become stem cells were bright!
- Single mutants seem normal—a little longer to grow things, but they catch up and do alright.
- Double mutants show normal protonema and gametophores; but no mRNA was detected at all. Fewer sporophytes… seemed to develop normal sexual organs…seem to fertilize okay (but don’t know for sure) but the zygote doesn’t grow and split in2 like normal WT.
- Even under 2 months of gametangia-inducing conditions, no sporangia.
- Some dbl mutants formed malformed porangia without normal spores… like parthenogenesis? (definition?)
- showed the gametes were OKAY by crossing with WT—92.5% success (WTxWT)vs 5.6 success (dbl mutant) vs 8.9%dblxWT) n=10
- re-grew some normal looking ones form dbl cross—one ended up having WT but 2 had all mutant alleles… so means they Can fertilize.
- Normal: divide, then grow. Some naturally fail to grow.
- Double mutant-> less cells that can grow tip (but dividing ok)
- Single mutant-> somewhere inbetween for a, but ok for b.
- 48 hours later, protonema reporters RM09 and RM55 were present in all divided cells, regardless of whether they continued to grow.
- Suggests ppWOX13L is required for cell growth, not division.
- In normal chloronema development, they cultured WT and mutant on high osmolarity medium. Appeared delayed; dodn’t branch, but split into 2 without growing
- But on normal medium, they look indistringuishable—suggests potential role in cell wall expansion
- – NOT DONE
Kofuji, R., & Hasebe, M. (2014). Eight types of stem cells in the life cycle of the moss Physcomitrella patens. Current Opinion in Plant Biology. 17: 13-21.
- physcomitrella patens has 8 stem cells
- need niche cells like in metazoan
- haploid bodies of flowering plants have reduced number of cells and have no stem cells (3,4)
- chloronema has apical cell and can branch form random cells, but we don’t know how yet (9)
- chloronemaà bright green; photosynth
- caulonemaà spindle, less green; for expansion
- gametophyte has apical cellà produced primordial cells for stem and leaves (25)
- unlike other plants, no callous forms
- wounding (53)
- no exogenous hytohormones; within 48 hours!
- Intrinsic auxin, cytokinin regulatory systems, transcriptional regulators change (55, 56, 57)
Nishiyama, T. Digital gene expression profiling by 5’end sequencing of cDNAs during reprogramming in the moss physcomitrella patens
- how to transform P. patens
- Schaefer 20à transformation procedures
- Schaefer, D. 1994, Molecular genetic approaches to the biology of the moss Physcomitrella patens [PhD Thesis], University of Lausanne, (http://www.unil.ch/lpc/docs/ DSThesis.htm)
Busch, H. Network theory inspired analysis of time-resolved expression data reveals key players guiding P. patens stem cell development PLoS one 2013 8:
Chopra, RN Biology of Bryophytes
Xiao
(Giles, K.L. (1971). Dedifferentiation and Regeneration in Bryophytes: A Selective Review. New Zealand J of Bota. if they stay attached, they will not dedifferentated
- if they stay attached; they don’t grow
- Mnium affineà only have 10-15 bud producing cells, but can form 100s of protonema
- Splachnumà can’t tell which ones will regenerate
- FUnaria hygrometricaà
- Some kind of innate ‘stability’ in whole plant that breaks down when you take leaves off
- Perhaps some sort of polarity? (Giles, von Maltzahn 1959)
- Suggests this because some parts of plant, like sporophyte, will better differentiate than other parts. Basal protonema tends to be better than apical (Westerdijk 1907)
- Von Wettstein (1924)à sporophyte is better at apical and decreases at basal
- Can also change on which direction you grow it via light
- Giles and von Maltzahn (1967; 1968)
- Different in different plants: Dawsonia don’t dedifferentiate but display chloroplast characteristics (Giles 1971)
Ishikawa
2011
Project Outline
TEMPLATE FOR PROJECT OUTLINE
Student’s name: Melissa Chen
Topic chosen: Leaf cell dedifferentiation in Bryophytes
SPECIFIC QUESTION:
What is the triggering mechanism that allows differentiated leaf cells in bryophytes (specifically, Funaria hygrometrica) to dedifferentiate and become identical to protonemal cells?
HYPOTHESIS:
The dedifferentiation of detached leaf cells is caused by the absence of certain cytoplasmic inhibitors in Funaria hygrometrica.
EVIDENCE ON WHICH THE HYPOTHESIS IS BASED (INCLUDE REFERENCES):
References are in annotated bibliography section.
PART 1: Regular protonemal development
Protonema is a totipotent-like state that emerges from germinating spores (Vanderpooten and Goffinet 2010). There are two stages: chloronema and caulonema. The chloronemtal stage precedes the caulonematal stage, and it has been shown that the two stages release different hormone factors into the environment (Bopp 2008, Brandes and Kende 1968). Factor H (which is secreted by caulonema) promotes bud formation, whereas Factor F (which is secreted by chloronema) inhibits bud formation. These factors are detected by neighbouring protonema in a concentration-dependant manner, and there are many cytokinin analogues that can produce similar effects.
PART2: Leaf dedifferentiation
It has been shown that fragments of bryophytes have the amazing ability to revert back into protonematal stages (chloronemal-identical stage) (Ishikawa et al. 2011). It seems that separating (by cutting, ripping, etc) leaves from the main stem will result in dedifferentiation, whereas attached-leaves usually do not tend form protonema (although there have been many notable exceptions) (Giles 1970). Some sources report that it is the cells near the ‘cut’ site (boundary cells) that ultimately sprout protonema (Ishikawa et al 2011), but personal observations have suggested that protonematal re-development occurs along the entire leaf lamina. It is unclear whether the dedifferentiation of boundary cells is unique to certain species (such as Physomeitralla) or situations.
PART3: What is known about the pathway and different hypothesis
It is known that a protein Cyclin-dependent kinase A (CDKA) is necessary for dedifferentiation of leaf cells into protonemal cells by causing leaf cells to re-enter the mitosis cycle. Since all cells in bryophytes are arrested in the S-phase of mitosis (unlike most other angiosperms, who are arrested in the G1-phase), the re-entering into the cell cycle is initiated by an asymmetrical division of leaf cells. This asymmetrical division will then give rise to an apical and basal cell—the former of which forms the first protonemal cell. Although CKDA is present in all gametophyte tissue, it must be activated by CDKD in order to promote cell division. Previous work by Ishikawa et al. (2011) has shown that boundary cells in Physomeitralla express CDKD, suggesting that injury to tissue somehow initiates cell dedifferentiation. Some sources suggest that leaf tissue in bryophytes possess an internal ‘polarity’ to them, which can be destroyed by eliminating the connection of the leaf to the stem (Giles 1970). It is also possible that gametophytic tissue excretes some type of hormone or signalling factor to repress protonemal growth in the gametophyte stage. Protonema already uses this system to signal to each other when to form gametophytic buds, so it is easy to see how such a system could be modified to perpetually supress protonemal growth. This model may also explain why leaves sometimes still dedifferentiate into protonema despite being attached to the main shoot. Lastly, it is possible that there is some ubiquitous factor within gametophytic cytoplasm that supresses the transcription of CDKD, and consequently the activity of CDKA. When tissue is damaged or cut, the loss of neighbouring cytoplasm—and consequently, repressing factors—may initiate CDKD transcription and cause dedifferentiation. This would explain by in-lab test have shown CDKD to be active only around cut sites, whereas other experiments have shown protonemal growth all over the leaf. The growth of protonema from all over the lamina could be due to small puncture wounds on the leaf when removing leaf tissue from the stem. This last model is the question I will be testing. I plan to either confirm or eliminate the possibility of cytoplasm repressive factors acting as the trigger for CDKD transcription, and ultimately cell dedifferentiation.
PREDICTION(S):
I predict that by removing cytoplasm from mid-lamina cells in a leaf that is still attached to the main shoot, I can induce cell dedifferentiation and resulting protonemal growth.
EXPERIMENTAL APPROACH TO TEST PREDICTION (INCLUDE ANY DETAILS THAT YOU HAVE WORKED OUT SO FAR):
First, obtain a transgenetic sample of Funaria hygrometrica that has a GFP reporter gene attached to the end of CDKD.
Next, I will attempt to induce protonemal development by removing the cytoplasm from lamina cells of leaves still attached to the main shoot. This will include 4 treatments:
- Whole leaves, detached from the main stem (positive control)
- Whole leaves, still attached to main stem (negative control)
- Whole leaves, still attached to main stem with mid-lamina cells punctured by a small needle (puncture control)
- Whole leaves, still attached to main stem, with mid-lamina cell cytoplasm removed with small needles.
All treatments will be cultured on Knop’s Agar (a common media for bryophyte cultures) and be kept under indirect sunlight at natural light cycles. I will be assessing the cultures for protonemal growth, speed of growth, and location of growth, as well as the presence of the GFP reporter gene. I expect the reporter gene will be present around the areas that protonema emerge.
If my hypothesis is correct, I expect to see protonemal growth at the cells directly adjacent to the cells that I choose to remove cytoplasm from. Additionally, I would expect to see no protonemal growth in the positive control or puncture control. This result would suggest that there is indeed some repressive factor in the cytoplasm that prevents spontaneous protonemal growth from occurring.
Some other possibilities would be if I saw the puncture controls growing protonema out of the punctured cells, but no protonema growing from the treatment with cytoplasm removed. This would instead suggest that cell injury causes some kind of protein cascade that ultimately causes CDKD expression.
Other interesting results may include protonema growing from cells that were neither punctured nor next to empty cells. Protonema may also grow on the positive control, which may suggest exogenous signals (hormones, gravity, nutrients, etc) at play.
LIST OF RELEVANT PRIMARY AND REVIEW ARTICLES READ, AND SUMMARY OF RELEVANT INFORMATION FROM EACH (this is the start of an annotated bibliography):
Brandes, H. & Kende, H. (1968). Studies on Cytokinin-Controlled Bud Formation in Moss Protonema. Plant Physiol, 43, 827-857.
This reference looks as the effect of various hormone analogues on protonema of Funaria hygrometrica. They show that some naturally produced hormones (called Factor H) induce bud formation on protonema, and that this can also be induced by adding cytokinins to the protonema. These hormones/cytokinins appear to be needed at a constant concentration in order to induce bud formation, and washing away these factors during bud development will stop development. Buds that have these factors removed will often revert back to protonema. They also show that it is possible to reverse bud formation by applying indole-3-acetic acid (IAA).
Szweykowska, A. (1961). Kinetin-induced formation of gametophores in dark cultures of Ceratodon purpureus. J. of Experimental Botany, 14(40), 137-141.
This reference shows that it is possible to induce bud formation in the dark by simple application of kinetin—which is crazy because one would assume light is an incredibly important energy source to have when developing gametic tissue.
Saunders, M.J., & Hepler, P.K. (1983). Calcium antagonists and calmodulin inhibitors block cytokinin-induced bud formation in Funaria. Developmental Biology, 99, 41-49.
This references adds onto the process of bud formation in mosses. They show that in Funaria hygrometrica, the cytokine-induced bud formation relies on the rise of intracellular calcium. It appears that calcium sources are from the outside of the cells, since calcium uptake inhibitors will prevent cell division, and consequently differentiation. This suggests that mosses are often reliant on exogenous signals to develop, and that nutrient availability may play a role in signalling to cells when to develop.
Bopp, M. (2008). Development of protonema and bud formation in mosses. J. Linn. Soc. (Bot.), 58(373), 305-309.
This reference shows that the hormones that protonema excrete work in a concentration-dependent manner, and that different factors are released at different stages to induce growth patterns. Protonema appear to inhibit each other, and thus promote a spread-out growth pattern. They identified two factors : Factor H (secreted from caulonema) which promotes bud formation, and Factor F (secreted from caulonema) which inhibits bud formation.
La Farge, C., Williams, K.H., & Engalnd, J.H. (2013). Regeneration of Little Ice Age bryophytes emerging from a polar glacier with implications of totipotency in extreme environments. PNAS, 110(24), 9839-9844.
This paper explores how mosses are able to germinate from gametophyte fragments that were previously frozen in glaciers. Most of the fragments appear completely dead—yet they are able to produce protonema!
Giles, K.L. (1970). Dedifferentiation and regeneration in Bryophytes: A Selective Review. New Zealand Journal of Botany, 9, 689-694.
This paper claims that leaf cells tend to de-differentiate when detached from the main plant, and that once leaves are isolated they seem to lose stability of differentiation. They report that chloroplast movement after 48 hours of isolation marks the beginning of de-differentiation. The chloroplasts will move to one side of the cell, causing elongation of the leaf cell. Isolated leaves have been reported to produce up to 100 secondary protonema.
Ishikawa, M., Murata, T., Sato, Yoshikatsu, Nishiyama, T, Hiwatashi, Y., Imai, A., Kimura, M., Sugimoto, Nagisa, Akita, Asaka, Oguri, Y., Friedman, W.E., Hasebe, M., & Kubo, M. (2011). Physomitrella cyclin-dependent kinase A links cell cycle reactivation to other cellular changes during reprogramming of leaf cells. The Plant Cell, 23, 2924-2938.
This paper shows the first molecular mechanism discovered in bryophytes involved in dedifferentiation of cells. First, they show that leaf cells are arrested in the S-phase by comparing the genomic content with other plants of similar genomic size. They use Physomitrella as a model organism. They show that when you cut leaves off Physomitrella, the cut edge will form cells that are indistinguishable from the apical cells of chloronemata. They found that a protein called CDKA is necessary for this cell cycle progression, and that it needs to be activated by CDKD.
Van der Poorten, A. & Goffinet, B. (2010). Introduction to Bryophytes. Cambridge University Press: New York.
This textbook is a general guide to mosses and liverworts. It is helpful in the general terminology and anatomy of bryophytes.
HOW DOES THE QUESTION FIT INTO THE BROADER PICTURE, AND WHAT IS ITS IMPACT?
Bryophytes can revert differentiated cells into a totipotent state, and this is an ability that is not only unique, but extremely impressive. By studying the mechanisms that allow bryophytes to de-differentiate, one could apply the same concepts to other models. There is still so much to learn about the significance of the bryophyte life cycle: Why are cells arrested in the S-stage of mitosis? Is this relevant to its regenerative abilities? Can we use similar transgenic protein cascades to control the potency of human cells?—and I believe that studying this mechanism will provide insight on different approaches to stem cell research and widen our knowledge of the wonderful diversity in developmental adaptations.
POTENTIAL WAYS TO MAKE YOUR QUESTION KNOWN TO THE PUBLIC AT LARGE (OR TO YOUR NON-BIOLOGIST FAMILY AND FRIENDS):
Talk about it! Sign up for every conference I can think of and just get the information out there. I doubt most people realize the strange S-phase characteristic of mosses, and I think lots of people would be convinced mosses are cool if they are just given the chance.
As for my non-biologist family or friends, I would frame my work in the perspective of ‘stem cell research in plant models’. By linking this research with something they are already aware of and consider ‘important’, it would be easier to get them excited about moss research!
ANY OTHER PARTS OF THE PROJECT COMPLETED SO FAR:
Not much else—I’m planning to work on it over the break!
ANYTHING YOU WOULD LIKE SPECIFIC FEEDBACK ON:
Would you like me to do a more experiments in this project? I could design experiments to test some of the other hypothesis I mentioned above, but wasn’t sure if you wanted something simple or something more comprehensive.
Proposal for Final Project
The Big Biological Question: What is the triggering event for new protonemal development in differentiated moss tissue?
Bryophytes are a group of early diverging land plants with ability to regenerate by fragmentation (Van der Poorten & Goffinet 2010). Under normal circumstances, bryophytes germinate from a spore as protonema, which are uniseriate filaments with totipotent capacity (Van der Poorten & Goffinet 2010). Gametophytic shoots then differentiate and bud from mature protonema and grow into the ubiquitous leafy structures by which mosses are known for. There is an apical cell in protonema and an apical region found in gametophytes that act analogously to stems cells, which allows new growth and differentiation (Prigge and Bezonilla 2010). The leafy gametophyte may give rise to structures specific for asexual or sexual reproduction, but surprisingly, cells in a variety of tissues also have the ability to grow entirely new plants (La Farge et al. 2013). New protonematal cells are able to bud from previously differentiated leaf, stem, or seta cells, which suggests that specialized tissue in bryophytes are able to dedifferentiated given certain conditions.
It has been shown that bryophyte cells are unique because unlike angiosperms, differentiated cells do not arrest in the G1 phase of the cell cycle. Rather, differentiated cells arrest in the S phase (Ishikawa et al. 2011). The proliferation of differentiated cells can be brought out of arrest by a CDKA1/CDKD pathway, which causes dedifferentiation through several cytosolic changes and the re-entering of the cell into a mitotic state (Ishikawa et al. 2011). These changes cause previously differentiated cells to resemble and behave like the immature protonema found post-germination. The protein CDKA1 is found in all tissues regardless of cell state or specialization, but is only active when interacting with CDKD (Ishikawa et al. 2011). In cases where moss leaves are cut and isolated from the main shoot, CDKD accumulates in the cells bordering the cut site within 48 hours, and these cells begin to stream their chloroplasts to one end (Ishikawa et al. 2011, Giles 1970). The streaming also causes the cell to bud, eventually dividing assymetrically into an apical cell (of the newly forming protonema) and a basal cell (Ishikawa et al. 2011, Giles 1970).
What I wish to investigate is the triggering event that causes transcription of CDKD. Some sources have suggested that the removal of a leaf causes cells to lose polarity, which may initiate cell dedifferentiation (Giles 1970). However, it is unclear if this is the case because there have also been reports of leaves giving rise to protonema while still attached to the main stem (Giles 1970). It is also possible that the loss or injury of cells causes some sort of deficiency on the connecting cell wall to neighbouring cells, which could trigger protonemal development. Lastly, perhaps there is some sort of exogenous control that triggers ‘stray’ leaves to produce protonema. In regular protonema, hormones that both inhibit and promote protonema maturation and bud formation are released from the protonema itself (Bopp 2008). Perhaps a similar mechanisms is used in bryophytes to control cell dedifferentiation.
My hypothesis is that CDKD production is controlled at a transcriptional level by endogenous chemicals produced by all differentiated cells. I suspect that these ‘endogenous chemicals’ under normal circumstances inhibit the transcription of CDKD, and the separation of leaf cells from the stem causes cells bordering the cut site to lose inhibition from these endogenous chemicals because of the loss of cytoplasmic content in some cells.
First, I will create a transgenic strain of Funaria hygrometrica that has a GFP reporter construct at the 3’ end of the CDKD gene, similar to the one found in Ishikawa et al. (2011). Next, I will attempt to induce protonemal development by removing the cytoplasmic content from a row of lamina cells in the leaf. My experiment will include 4 treatments, all placed on Knop’s agar (a common bryophyte nutrient medium) under standard sunlight conditions.
- Whole leaves, detached from the main stem (positive control)
- Whole leaves, still attached to main stem (negative control)
- Whole leaves, still attached to main stem with mid-lamina cells punctured by a small needle (puncture control)
- Whole leaves, still attached to main stem, with mid-lamina cell cytoplasm removed with small needles.
I expect that the leaves detached from the main stem will produce protonemal buds within 48 hours, whereas whole leave that remained attached to the main stem will not produce any protonemal buds. The puncture-control leaves are expected not to produce protonema. Finally, the whole leaves with cytoplasmic content removed are expected to produce protonemal buds to form around the border of the ‘empty’ cells due to the lack of neighbouring inhibition of CDKD. I will also view all samples under a UV light to visualize the distribution of CDKD. I expect CDKD to be produced in all the cells previously predicted to grow protonema.
Although overlooked, bryophytes are an extraordinary group of organisms that have adapted to be able to clone itself by mere fragmentation—a feat that most other organisms cannot do. Not only this, but it appears its reproduction can originate from any and all living tissue and does not require a pre-specified meristem or stem cell. By investigating the mechanism behind the activation of dedifferentiation, one can apply the concepts harnessed by bryophytes and hopefully understand some of the regulating pathways found in other organisms. It is possible that these ideas may be applicable to human stem cell research in the future.
References:
Bopp, M. (2008). Development of protonema and bud formation in mosses. J. Linn. Soc. (Bot.), 58(373), 305-309.
Giles, K.L. (1970). Dedifferentiation and regeneration in Bryophytes: A Selective Review. New Zealand Journal of Botany, 9, 689-694.
Ishikawa, M., Murata, T., Sato, Yoshikatsu, Nishiyama, T, Hiwatashi, Y., Imai, A., Kimura, M., Sugimoto, Nagisa, Akita, Asaka, Oguri, Y., Friedman, W.E., Hasebe, M., & Kubo, M. (2011). Physomitrella cyclin-dependent kinase A links cell cycle reactivation to other cellular changes during reprogramming of leaf cells. The Plant Cell, 23, 2924-2938.
La Farge, C., Williams, K.H., & Engalnd, J.H. (2013). Regeneration of Little Ice Age bryophytes emerging from a polar glacier with implications of totipotency in extreme environments. PNAS, 110(24), 9839-9844.
Van der Poorten, A. & Goffinet, B. (2010). Introduction to Bryophytes. Cambridge University Press: New York.
Assignment 1
- Are the cells found in the protonema of bryophytes all considered pluripotent, or are there particular sections of the protonema destined to become differentiated into the gametophyte stage? Furthermore, how does Methylobacterium induce faster growth of protonema in Funaria ? Does it prevent further cell differentiation, or does it simply increase nutrient availability?
- How similar is the development of stomata on tracheophyte sporophytes and the stomata on bryophyte sporangia? Are they homologous, or are they converging?
- What structural/biochemical mechanisms surround Paramecium aureli’s ability to change its tolerance to heat, salt, and arsenic, and why does this tolerance disappear after several generations?
As a bryo-enthusiast, I would love to be able to study the development, history, and relevance of bryophytes to humans because I think they are amazing organisms that deserve far more credit that they receive. One of the things that attracts me to them is their simplicity and the way they are able to thrive in such diverse habitats despite having relatively simple structure, physiology, and development. Furthermore, I am particularly interested in looking at host-microbe relationships. Considering we know so little about the complex microbial world and how communities of microbes change ( or are changed by) other organisms, I believe this topic is a marriage of my two passions.
If the results of my potential experiment showed that protonema (specifically, caulonema, which is the stage of protonema right before gametophyte and rhizoid differentiation) consisted of a large collection of identical cells (that is, there is no pre-determined stem cell from which the gametophyte grows), it would suggest that there are some factors or signals that induce further differentiation. I believe this would be fascinating to the world of developmental genetics because it would be an example of how plants signal cell differentiation and specificity. conversely, if it is revealed that the point of cell differentiation is pre-determined in the early protonemal stages, I could investigate what causes particular cells to be ‘chosen’ and how one might manipulate this.
Additionally, I would want to investigate the effect of Methylobacterium (a protonema-associated microbe) has on protonemal growth. I would want to investigate why Methylobacterium-associated hosts tend to grow more extensive protonema– for example, whether it grows faster because of increased induction of growth genes, of if it is because there is a repression of differentiation genes. This could be important in understanding how to induce faster stem cell growth or how to prevent stem cells from further differentiating. Either option could provide useful knowledge about how to manipulate cell growth and differentiation, and it may lead to us using Methylobacterium as a tool to study stem cells in other organisms.
In the world of science, the ability to understand how plants sustain pluripotent cell stages (like protonema), what triggers differentiation, and what external factors may prevent differentiation could be used in many different fields. Cell cultures used for other experiments could be sustained better due to increased knowledge of how to prolong cell stages. Experimental organisms could be grown at faster rates if we were to understand how to speed up the development process. In society, we may even be able to extend the tools and concepts gained from these results into treating human disease and illness. For example, if studies on Methylobacterium were to reveal that the bacteria produced a compound that prevents the expression of certain genes in development, one could potentially use similar compounds in tumour therapy to control rapidly dividing cells. Although my proposed question may not directly affect society, there are many ways its results could influence new ideas that would eventually lead to better medicinal practices.