The objective of the Definition Assignment was to choose a word that relates to my professional field and define it for a particular audience. I decided to define the word ‘fuel cell’ and explain it to a nontechnical audience. Three definitions of the term ‘fuel cell’ are included: a parenthetical, a sentence, and an expanded definition. In my expanded definition, I elaborate on the benefits, uses, safety concerns, and components of fuel cells.
Introduction
Definitions are important in technical communication to convey information. The purpose of this assignment is to inform non-technical readers about the function of a fuel cell, from the perspective of a company that specializes in fuel cell production. These readers will need an overview of the device, how it works, its benefits, current applications, and safety concerns.
The fuel cell function and uses will be explained with the use of a parenthetical definition, a sentence definition, and an expanded definition.
Parenthetical Definition
With the negative impact climate change has on the world, fuel cells (hydrogen powered devices that produce electricity) have become increasingly important to combat the growing climate crisis.
Sentence Definition
A fuel cell is a device that produces electricity via an electrochemical reaction with hydrogen and oxygen. With similar uses as traditional batteries, fuel cells can be used in applications including: transportation and infrastructure (“Fuel Cell Basics”).
Expanded Definition
What is a fuel cell?
A fuel cell is a device that conducts energy, similar to a battery. However, unlike traditional batteries that use metals to conduct electricity, fuel cells generate electricity through an electrochemical reaction with hydrogen and oxygen gas (“Fuel Cell Basics”). Fuel cells do not store energy, so they need a constant supply of hydrogen to produce electricity.
How does a fuel cell work?
Fuel cells contain a positive electrode (cathode) and a negative electrode (anode). Between these two electrodes lies an electrolyte membrane (“Fuel Cells”). As seen in Figure 1, a fuel cell operates by flowing hydrogen into the anode side and oxygen on the cathode side. As hydrogen and oxygen enter the cell, they react across the membrane (“Fuel Cell Basics”). Small particles called electrons are stripped from hydrogen and used to create electricity by flowing through an external circuit (“Hydrogen Facts”). The hydrogen then combines with the oxygen to form water. The overall electrochemical reaction that occurs in a fuel cell releases heat, water, and electrons.
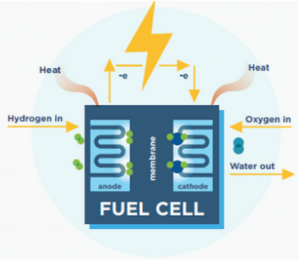 Figure 1. Fuel cell parts and operation (“Fuel Cell Basics”).
Figure 1. Fuel cell parts and operation (“Fuel Cell Basics”).
What are the benefits of fuel cells?
Fuel cells are efficient, environmentally friendly, and affordable (“Fuel Cell Electric”). Whereas traditional combustion engines emit carbon dioxide, fuel cells are a low or zero emission alternative that can mitigate the climate crisis (“Fuel Cells”). With few moving parts, fuel cells remain quiet during operation (“Fuel Cell Basics”). Fuel cells are also scalable, as they can be stacked together to power larger systems.
How can fuel cells be used?
Fuel cell technology is currently used in commercial vehicles as a clean energy alternative to combustion engines. Compared to electric vehicles, fuel cell vehicles can be quickly refuelled and have a larger driving range (“Hydrogen Facts”). Other applications for fuel cells include: energy storage, electronics, transportation, and more (“Fuel Cell Basics”).
Are fuel cells safe?
Fuel cells are generally considered safe. However, there are some safety concerns that are shared with batteries and combustion engines. As fuel cells operate like batteries, high currents are generated, which can risk electrical shock (“Safety Issues”). Additionally, although hydrogen is a non-toxic fuel, it is a flammable gas (“Safety Issues”).
References
“Fuel Cells.” Energy.gov, https://www.energy.gov/eere/fuelcells/fuel-cells.
“Fuel Cell Basics.” Fuel Cell & Hydrogen Energy Association, https://www.fchea.org/fuelcells
“Fuel Cell Electric Vehicles.” Alternative Fuels Data Center: Fuel Cell Electric Vehicles, https://afdc.energy.gov/vehicles/fuel_cell.html
“Hydrogen Facts.” Hydrogen Facts | About Ballard | Ballard Power, https://www.ballard.com/about-ballard/hydrogen
“Safety Issues Regarding Fuel Cell Vehicles and Hydrogen Fueled Vehicles.” Minnesota Department of Public Safety, https://dps.mn.gov/divisions/sfm/programs-services/Documents/Responder%20Safety/Alternative%20Fuels/FuelCellHydrogenFuelVehicleSafety.pdf#search=fuel%20cell%20safety.
