As atmospheric carbon levels continue to rise, some scientists have
recommended a potential solution–pouring acidified iron sulfate into
the ocean (Powell, “Will Ocean Iron Fertilization Work?” 2008). For
some, the idea of dumping chemicals into the ocean to fix a problem
with the atmosphere may seem puzzling. However, there is some
scientific reasoning behind this idea.
Some areas of the ocean are high in most nutrients, but critically low in
iron. This lack of iron limits the growth of phytoplankton in these
otherwise nutrient-rich areas. Since phytoplankton absorb atmospheric
carbon for photosynthesis, this lack of iron also limits the rate of
atmospheric carbon being lost to the ocean (Powell, “Will Ocean Iron
Fertilization Work?” 2008).
The ‘iron hypothesis’ suggests that by fertilizing these areas with iron,
phytoplankton can increase in abundance and more atmospheric
carbon will be lost for their photosynthetic purposes (qtd. in Cao and
Caldeira 2010). And when the phytoplankton die, they simply sink and
carry the absorbed carbon to the ocean floor–where it remains for
decades to millennia (Wayman 2008).
However before people starts dumping chemicals into the ocean, it is important to important to consider
any potential effects to marine life.
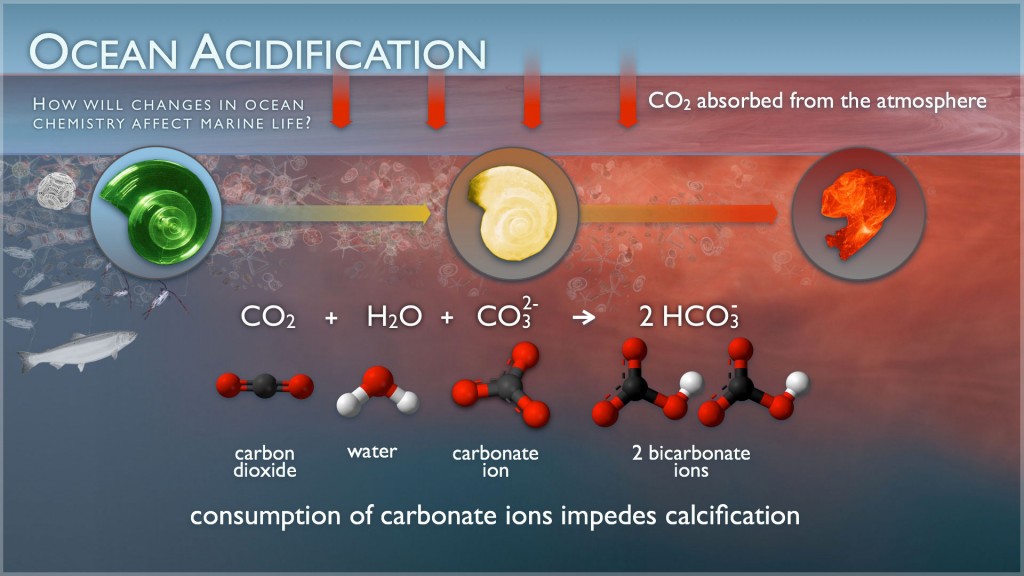
The increased amount of carbon brought to the ocean by iron fertilization can affect the chemistry of
seawater, leading to ocean acidification. This acidification could jeopardize the lives of many marine
animals by impairing their calcifying abilities, which are needed to maintain exoskeletons like the snail
shell pictured below (Cao and Caldeira 2010; Zeebe et. al 2008).
Phytoplankton booms can also block valuable sunlight from reaching certain organisms. One such group
of organisms would include the corals, which require sunlight to grow (Powell, “Fertilizing the Ocean with
Iron” 2008).
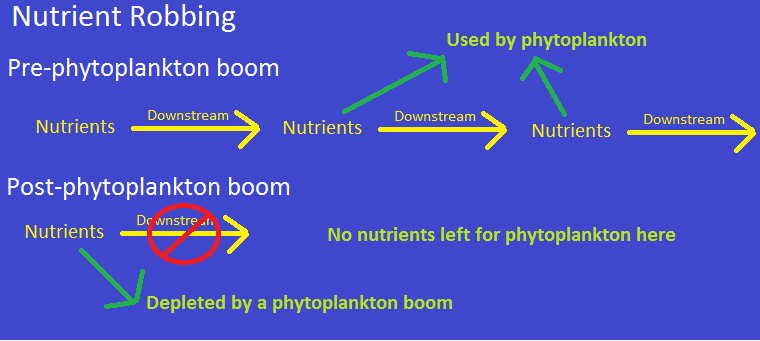
Sometimes, iron fertilization can also do the opposite of what it is intended to do. One example of this is
known as ‘nutrient robbing’. Phytoplankton blooms deplete certain nutrients which would have washed
downstream (if not for iron fertilization). Without these nutrients arriving downstream, the number of
downstream photosynthetic organisms would decrease (Oschlies et. al 2010). In other words, iron
fertilization could decrease the number of carbon absorbers in some unfertilized areas.
Phytoplankton also produce the greenhouse gases methane and nitrogen dioxide (Powell, “Fertilizing the
Ocean with Iron” 2008). So although phytoplankton blooms could absorb atmospheric carbon, they
release other problematic gases right back into the atmosphere (Wayman 2008).
The effectiveness of iron fertilization is also questionable because sometimes zooplankton eat blooming
phytoplankton as fast they appear (Powell, “Fertilizing the Ocean with Iron” 2008). In the summer of
2012, a patch of water near Haida Gwaii was fertilized with iron. This caused a phytoplankton bloom, but
by autumn, it was gone. Since crustacean zooplankton numbers were very high, they likely devoured
these new phytoplankton almost immediately (Batten and Gower 2014).
Although it is great to see people try to address the problem of rising atmospheric carbon levels, iron
fertilization has way too many downfalls and is by no means a proper solution.
Work Cited
Batten, SD & Gower, JFR (2014). Did the iron fertilization near Haida Gwaii in 2012 affect the pelagic lower trophic level ecosystem?. J Plankton Res36.
Cao, L & Caldeira, K (2010). Can ocean iron fertilization mitigate ocean acidification?. Clim Change99.
Haiken, M (2007). Fertilizing Oceans to Save the Planet. CNN Money.
Oschlies, A, Koeve, W, Rickels, W & Rehdanz, K (2010). Side effects and accounting aspects of hypothetical large-scale Southern Ocean iron fertilization. Biogeosciences7.
PMEL Carbon Group (2014). Ocean Acidification: The Other Carbon Dioxide Problem 2014.
Powell, H (2008). Fertilizing the Ocean with Iron. Oceanus46.
Powell, H (2008). Will Ocean Iron Fertilization Work?. Oceanus46.
Wayman, E (2008). Seeding the Sea. Geotimes53.
Zeebe, RE, Zachos, JC, Caldeira, K & Tyrrell, T (2008). Oceans. Carbon emissions and acidification. Science (New York, N.Y.)321.