Introduction
The criteria of this assignment is to chose a term within my discipline and provide a parenthetical definition, sentence definition, and expanded definition. The objectives for this assignment are to understand the need for defining terms in technical writing, differentiate between the ways of defining terms, and learn how to use them appropriately. For this assignment it was assumed that the reader would have a basic knowledge of scientific terms such as electron and photon.
Parenthetical definition
The photoelectric effect (electron ejection caused by light) created doubt about the theory that light only behaved like a wave.
Sentence Definition
The photoelectric effect is the phenomena that occurs when an electron is ejected from a material after it is exposed to light. If the energy that the electron absorbs from the photon is large enough, the electron will be ejected.
Expanded Definition
History
The photoelectric effect was first observed by Heinrich Hertz in 1887 (“Photoelectric Effect”). The Photoelectric Effect describes how Hertz saw that ultraviolet light changed the voltage for which sparking took place between two metal electrodes (an electrical conductor). In 1902, Philipp Lenard showed the relationship between light and electricity; when a metal is lit electrically charged particles (electrons) are emitted (“Photoelectric Effect”). This event of light illuminating a material and photoelectrons being emitted is known as the photoelectric effect.
Relation to Light
It was expected that the energy caused by the movement of the electron emitted by the effect would by proportional to the intensity of the light used, however this was found not to be the case (“Photoelectric Effect”). It is proportional to the frequency of the light (which determines its colour), and intensity determines the number of electrons that is emitted (“Photoelectric Effect”).
Diagrams of the effect
The image below represents the photoelectric effect. The image is of different frequencies of light being shone at a metal plate (Elert). The light with higher frequencies produce photoelectrons with higher kinetic energy than light with low frequency does (Elert).
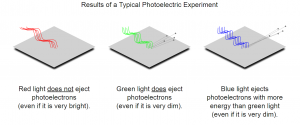
Figure 1: three experiments using lights of different frequency (Elert).
Uses
The photoelectric effect has a variety of uses in lab settings, as well in industry. A device that utilizes the photoelectric effect is the photodiode. Photodiodes can detect light and measure its intensity, as well as turn light into electrical energy (“Photoelectric Effect,”). Some examples of the applications of photodiodes are described by Britannica as pollution monitoring, imaging, solar cells, and fibre optics (“Photoelectric Effect”). Automatic doors can use photodiodes as their sensor. A light is shone at a photodiode and when that light is interrupted, usually by a person walking through it, the photodiode sends a signal that opens the door (“Principle of Sensor Operation”).
References
“Photoelectric Effect.” Encyclopedia Britannica Online. Encyclopedia Britannica, n.d. Web. https://www.britannica.com/science/photoelectric-effect Accessed: 26 September 2016.
“Principle of Sensor Operation” OPTEX. n.d. Web. http://www.optex.co.jp/as/eng/infrared/principle.html Accessed: 26 September 2016.
Elert, Glenn. “Photoelectric Effect.” The Physics Hypertextbook. n.d. Web. http://physics.info/photoelectric/ Accessed: 26 September 2016.
File of the assignment:
sarah-parry-definition-assignment
Samuel Navarro’s Review of my assignment:
http://engl301.arts.ubc.ca/2016/09/28/peer-review-of-sarah-parrys-definitions-assignment/
