I think that for technology to be effectively used in the classroom (science or otherwise), technology should enhance or add opportunities for learning. For example, considering the use of a computer to type up a lab report rather than writing it by hand may be neater, but is not enhancing the learning opportunity. On the other hand, the use of digital reflections helps to enhance learning by increasing the opportunity for students to share ideas in a community of learning. Digital simulations may allow students the chance to explore situations not accessible in a regular classroom such as theoretical physics concepts or modelling the invisible molecules of life or a chemical reaction or an aspect of gene control. PhET has some excellent simulations that will help to address some misconceptions such as the mass of gases, or conservation of mass in a reaction. There is nothing wrong with using technology for its own sake, but students are so constantly exposed to tech, that I believe there should be a justified use of tech on the basis of contributing something beyond the regular experience. There are many online websites and activities that students can explore at their own pace, supporting differentiated learning or providing a sense of a lab scenario that would be possible in a classroom setting. DNAi.org is a website dedicated to genetics with video clips, historical background and activities exploring our understanding of DNA and genetics processes and how we learned this. NASA for Kids is another example of student-driven exploration of the solar system. Other examples is using tech to extend student work such as the use of e-portfolios, video or animation creation, or course wikis. This past year, I was able to obtain laptops for my classroom, and students can now watch videos at their own pace (rewinding or pausing as necessary), participate in a learning community using reflections/discussions, a knowledge forum, wiki contributions, and a digital artifact assignment, as well as contributing to daily lessons through collaborative slide shows or presentations. To me these types of activities extend the learning, rather than maintaining status quo but with a screen.
Author Archives: david dykstra
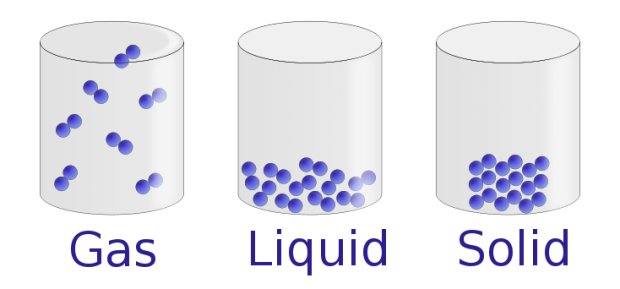
Does gas have mass?
In 1968, David Ausubel wrote “The most important single factor influencing learning is what the learner already knows. Ascertain this and teach him accordingly.” (Ausubel, 1968, p. vi). Unlike an earlier idea that learner are blank slates that need to be filled by the teacher, more recently, the Personal Construct Theory recognizes that the person is a meaning-make, capable being an active participant in their learning, and of making judgements about their interpretations of evidences (Shapiro, 1988). If students enter a topic or concept with various misconceptions or alternative frameworks, this will negatively influence both their ability and willingness to incorporate new understandings. In science, this is quite prevalent, perhaps because we first use simple analogies to explain a concept and then elaborate more fully later, or perhaps the nature of science allows children to make their own observations and interpretations first. Whatever the reason, many students have misconceptions about various aspects of science. For example, many of my Bio11 students believe penguins have fur not feathers!
I have chosen to work with the misconception that gases don’t have mass, or even have a negative mass. This common misconception is likely due to students observations that adding more gas to a helium balloon causes it to float higher (Mayer, 2011), or trapping air bubbles under a toy in the bathtub allows it to float instead of sinking. According to Mayer, (2011), even after students performed an experiment in a sealed flask demonstrating that the mass doesn’t change when a substance (iodine) goes from a solid to a gas, the majority still stuck to their false preconceptions and assumed they must have made an error in the experiment. This lead Mayer to recognize that preconceptions are very difficult to remove (Mayer, 2011). This phenomenon was also seen in the video clip about Heather and how she continued to stick to her theories about the strange curlicue orbit of earth and how light bounces to give indirect light.
So what do we do about these misconceptions. Elliot (2016) notes that teachers who are aware of common misconceptions among their students were more effective, and that the common teacher approaches of avoiding students’ existing beliefs altogether or telling students clearly they are wrong and that they need to think differently are ineffective. Posner et al (1982) view learning as a “process of conceptual change” where accommodation of misconceptions is needed to reorganize their current beliefs. For accommodation to occur, learners need to be dissatisfied with their current idea or model, and the new model must be rational, intelligible, plausible, and fruitful for further research (Posner et al., 1982). Teachers can use anomalies to point out limitations of misconceptions, thereby bringing the cognitive conflict (dissatisfaction) that drives new understandings (Elliot, 2016, Posner et al., 1982). I would suggest that using technology is a good way to do this for many abstract concepts. There are many online simulations (for example PhET) that enable learners to view the concepts, play with them, and visualize the greater explanatory ability of the scientific idea such as the states of matter and properties of gases (https://phet.colorado.edu/en/simulation/states-of-matter).
- Ausubel, D.P. (1968). Educational Psychology: A Cognitive View. New York: Holt, Rinehart & Winston.
- Elliott, K., & Pillman, A. (2016). Making science misconceptions work for us.Teaching Science, 62(1), 36-39.
- Harvard-Smithsonian Center for Astrophysics. (1987). A private universe. ISBN: 1-57680-404-6. Retrieved from http://learner.org/vod/vod_window.html?pid=9
- Mayer, K. (2011). Addressing students’ misconceptions about gases, mass, and composition. Journal of Chemical Education, 88(1), 111.
- Posner, G. J., Strike, K. A., Hewson, P. W. and Gertzog, W. A. (1982). Accommodation of a scientific conception: Toward a theory of conceptual change. Sci. Ed., 66: 211–227. doi: 10.1002/sce.373066020.
- Shapiro, B. L. (1988). What children bring to light: Towards understanding what the primary school science learner is trying to do. Developments and dilemmas in science education, 96-120.
Water in the Hard Drive
The first computer we had was a Commodore128 (not the 64 – this was TWICE as good – don’t ask me why!) which we used almost exclusively for games. Centipede, Alien Invasion, and SkiRun were favourites for those who remember… but my favourite was a football game. I played it every chance I got, but one day it didn’t work, instead an error message popped up saying “there is water in the hard drive, press D to drain”. After pressing D there was a gurgling noise, and another message “failure to drain, please contact company for help”. My first emergency response was to go to my oldest brother (our “expert” on the computer) only to have him almost bust his gut laughing – he had changed the file name and programmed those messages. That was when I first realized computers could be programmed by regular people, but I think I also decided then that it wasn’t for me. I have always relied on others to help with computers problems, beginning with my oldest brother. It is only since I started MET that I have begun to realize that I can manipulate technology too!