With the advent of gene modification and the ability to produce transgenic animals, medical research renewed its interest in xenotransplantation. Increased knowledge in the field of immunology allowed for the identification of specific antigens involved in immune responses involved in rejection, and knowledge in genetics allowed for the identification of endogenous retroviruses.
Genetic Modification & Production of Transgenic Animals
Production of transgenic animals began with two key discoveries
1. Genetic alteration by Homologous Recombination (HR) (figure 1)
2. The Embryonic Stem (ES) Cell System (figure 2)
The procedure:
- Harvest ES cells from a developing blastocyst
- Genetic information is delivered to ES cells by DNA microinjection, or by electroporation
- Genetic information is integrated into ES genome by HR
- HR is a naturally occurring phenomenon in developing zygotes— parental genetic information is recombined at homologous sites to give rise to genetically distinct offspring
- ES cells are implanted in a blastocyst of a surrogate female
- Some offspring will inherit the introduced gene
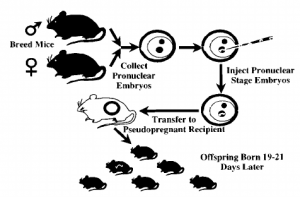
Figure 2. Production of Transgenic Mice by Embryonic Stem Cell System and DNA microinjection. (Roths et al., 1999)
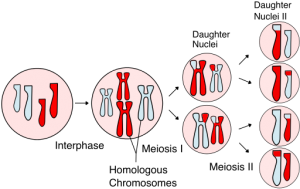
Figure 1. Homologous recombination giving rise to genetically distinct daughter cells.
CRISPR/Cas-9
- Clustered regularly interspaced short palindromic repeats (CRISPR) associated Cas-9 is a more recent technique for genetic engineering
- Gene modification by HR was unpredictable and inefficient
- CRISPR/Cas-9 allows for multiple mutations with high specificity in a single step
- Uses an endonuclease and a segment of guide RNA to precisely edit the genome (Figure 3)

Figure 3. CRISPR/Cas-9 Mechanism for Gene Editing
Genetic Engineering to Overcome Immunological Barriers
Improving immuno-compatibility of xenografts
- Targeting specific antigens on porcine cells that induce an immune response:
- galactose-a1,3-galactose (Gal) — oligosaccharide expressed on the surface of porcine cells was determined to be the most important antigen inducing recipient immune response
- a1,3-galactosyltransferase gene knockout (GTKO) pigs were produced by “knocking out” (eliminating) the gene responsible for Gal expression
Incorporation of human complement-pathway regulatory proteins:
- CD46, CD55 were “knocked in” (introduced) to GTKO pigs to reduce hyperacute rejection
- T-cell costimulation blockade agent, CTLA4-Ig and human major histocompatibility complex (MHC) were knocked in to reduce acute humoral xenograft rejection
- “humanized” xenografts— immune cells more likely to recognize foreign cells as human cells (no immune response engaged)

Strategy proposed for preventing pig kidney rejection in human recipient using pigs expressing human complement genes paired with pig bone marrow transfusion by Dr. David H. Sachs. (Flickr user Mnorri)
Removing Porcine Endogenous Retroviruses (PERVs)
- Using CRISPR, researchers have successfully inactivated all PERVs embedded within the pig genome in one step