Climate change is the change of weather and the rise of sea levels on the planet Earth. Climate change is an extremely relevant global issue since it can lead to flooding and extreme weather conditions which can endanger life on earth. As a result, it is of utmost importance to find solutions that can help mitigate the effects of climate change. One of the main causes of climate change is the release of excess carbon dioxide into the atmosphere, due to the burning of fossil fuels.

Image: Climate Change
Source: CC0 Public Domain
A solution to climate change
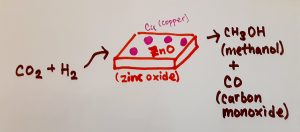
One solution that scientists have proposed in order to reduce the amount of atmospheric carbon dioxide is to capture carbon dioxide in the air and use the captured carbon dioxide as a source of chemical carbon for other processes. This process is known as “carbon capture and utilization” (CCU). Although the potential benefits of CCU are very promising, changing the carbon dioxide into a different form and using it in other chemical processes has been proven to be difficult, mainly due to the thermodynamic stability of carbon dioxide. Although CCU has gained major traction over the past few years, it will still require a lot of time before it can be used industrially worldwide. Scientists are currently in the process of trying to find the least costly, and most efficient means of capturing carbon emissions to reduce climate change.
 Video: Carbon Capture Plant in Squamish, BC
Video: Carbon Capture Plant in Squamish, BC
Carbon capture methods
One of the carbon capturing methods that has been showing promise in recent scientific studies, is the adsorption of carbon dioxide through the use of solid sorbents. Adsorption is the adhesion or the clinging of gas molecules onto a surface. In this case, the carbon dioxide molecules will stick to the solid surface of the sorbent, which leads to successful carbon capturing. The solid sorbents used in this method can be made of “porous carbonaceous materials, zeolites, alumina, silica, (or) metal-organic frameworks.” Adsorption of carbon dioxide can be categorized into two variations; physical and chemical adsorption. In physical adsorption, the transfer of carbon dioxide into the solid sorbent occurs due to the van der Waals interactions between the sorbent and the carbon dioxide. The issue with these physical sorbents is that they have “poor selectivity for CO2, and low CO2 adsorption capacities.” A means of improving both the carbon dioxide selectivity and the carbon dioxide adsorption capacities of these sorbents is by adding basic groups to the sorbent surface, which can strengthen its interactions with the acidic carbon dioxide. These sorbents primarily use alkalis to act as basic groups. In terms of alkali-based sorbents, scientists have been favouring the use of potassium carbonate and sodium carbonate. Although carbon dioxide absorption via solid sorbents is very promising, more scientific work needs to be done to improve the adsorption capabilities of sorbents.
Another carbon capturing method that scientists have been favouring is the separation of carbon dioxide via membranes. These membranes are selectively permeable to carbon dioxide which leads to separation of carbon dioxide from other chemicals.
All in all, the development of these innovative carbon capturing mechanisms is helping to mitigate climate change and scientists are working hard to refine these techniques.
– Yoshinao Matsubara