Did you know that excess carbon dioxide poses a significant problem for marine life? Although CO2 is naturally occurring and acts as an important heat-trapping gas in moderate amounts, human activities have pumped lots of excess CO2 into the atmosphere.

Exhaust fumes from an industrial plant, which includes carbon dioxide (as well as other chemicals). Photo by Damian Bakarcic.
Too much carbon dioxide not only contributes to more extreme weather and global warming, but it also made oceans 30% more acidic since the beginning of the Industrial Revolution.
Today, CO2 makes up 84% of all greenhouse gases from human activity, with around 40 billion tons being produced per year.
Numerous climate scientists, such as climatologist Dr. James Hansen, state that to avoid the impacts of climate change, the levels need to be reduced to at maximum 350 ppm. However, CO2 levels have already exceeded 400 ppm in 2019.
Across the world, many different marine species, such as barnacles, experience the chemical effects of lowered pH levels. This comes in the form of problems with shell formation/ adhesion and lower survival rates, as demonstrated in a laboratory study by the Northeast Coastal Acidification Network.
Ocean acidification has an especially adverse impact for animals that are sensitive to changes in carbonate chemistry. For example, shellfish use carbonate in the ocean to make their protective shell structures. With a low pH, calcium carbonate is in short supply because it will react with acidic solutions.
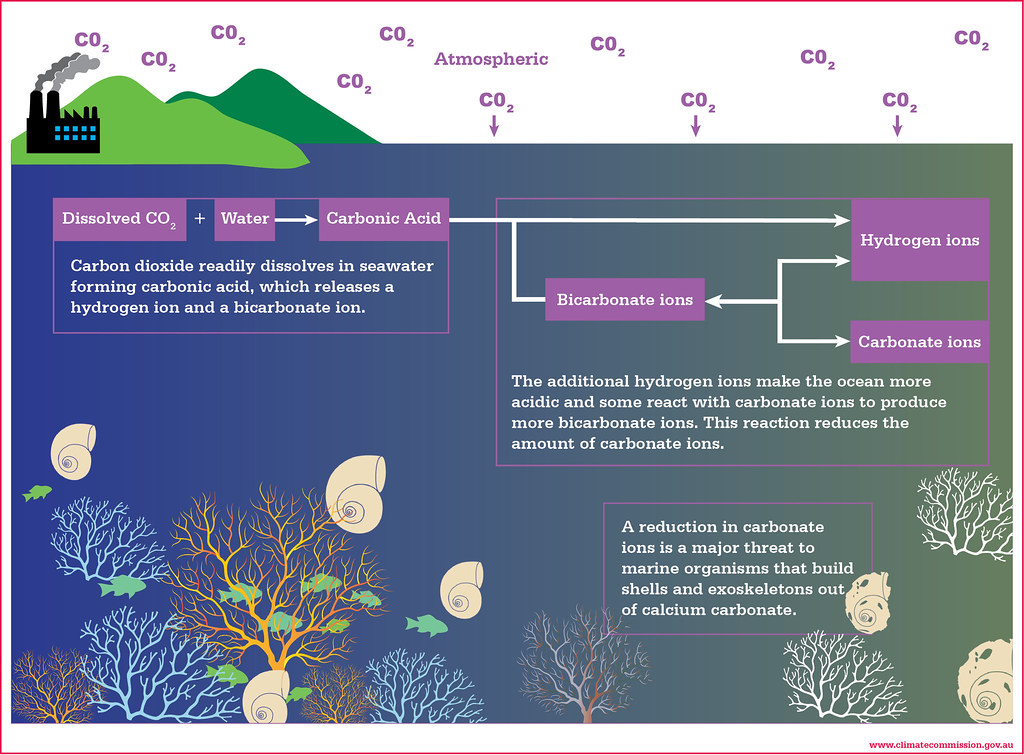
An infographic depicting the chemistry behind ocean acidification, and why it is harmful for marine life. Source: Climate Commission (RIP)
One remedy involves catalysis. This uses catalysts to convert CO2 into useful goods – fertilizers and plastics. Doing so would convert the polluting waste product into useful molecules, and simultaneously lessen our need to use fossil fuels to generate such products.
A way this can be achieved is with an electrolysis cell, which employs electrical energy to run a non-spontaneous redox (i.e. oxidation-reduction) reaction. A non-spontaneous redox reaction occurs only when an external voltage is applied, whereas a spontaneous one would generate a voltage itself.
On the electrode surface, the CO2 is reduced – meaning the addition of hydrogen, the removal of oxygen, or both (oxidation is the opposite: lose hydrogen/ gain oxygen). Depending on the number of electrons transferred, many different molecules could be produced. The products form in the electrolyte, and move to a separation system.
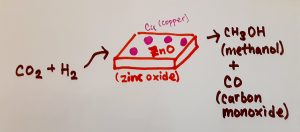
Catalysis involving carbon dioxide and hydrogen, with a Cu/ZnO catalyst. CO2 is reduced, producing methanol and carbon monoxide. Source: a drawing by myself.
Any unreacted CO2 and the electrolyte are recycled. Tin is a metallic catalyst used to make formic acid via catalysis. More complex molecules can be formed as well, such as the ethanol found in hand sanitizers.
In conclusion, too much carbon dioxide is a significant threat to marine life, and catalysis is one solution that scientists are investigating to recycle CO2. Are there other potential solutions for excess CO2 that you know of?
– Jacqueline (Wai Ting) Chan
