Whenever you take a shower, do you ever wonder how exactly soap works to clean you?

Soap. (Photo Source: “Soap Suds” by Steve Buissinne on www.publicdomainpictures.net)
I found it fascinating when I found out that soap was actually made from oils and fats. It was even more fascinating when I made my own soap in one of my chemistry courses, utilizing fats that can be found commonly in any household, such as olive oil, lard, and butter (ever recall seeing cocoa butter soap on your local grocery store?).

Butter. (Photo Source: “A stick of western pack butter” by Steve Karg on www.commons.wikimedia.org, This file is licensed under the Creative Commons Attribution 2.5 Generic license. No changes were made to this file)
You may be thinking “But wouldn’t soaps just make your hands more oily?”. It’s obvious that something has had to happen to the fat in order to make the soap able to pick up dirt and other oils, along with being able to wash away with water. To explain this, we must learn a little bit of organic chemistry.
Fats are non-polar molecules which generally consist of 3 long chains of carbon molecules, which are called “fatty acids“, attached to glycerol (on the image below, glycerol is the left side of the fat molecule up to the carbon with the double bonded oxygen).
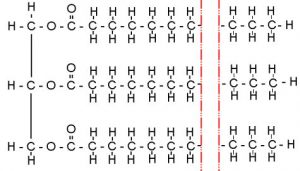
A fat molecule. (Photo Source: “Saturated Fat” by Louis Shackleton on www.flickr.com)
With the use of a strong base, such as soda lye (aka NaOH or caustic soda), the glycerol is removed from the fatty acids, which separates the fatty acids from one another and leaves them with a negatively charged oxygen in place of where the glycerol formerly was. From this, the chemical reaction with lye (known as ‘ester saponification‘) leaves the fatty acid with a non-polar carbon chain and a polar carboxylate end (the end with the negatively charged oxygen).
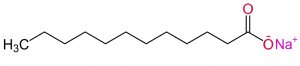
Example of a “soap” molecule with described structures. (Photo Source: “Sodium laurate” by Claudio Pistilli on www.commons.wikimedia.org, This file is licensed under the Creative Commons Attribution-Share Alike 4.0 International license. No changes were made to this file)
Due to this attribute of being polar on one side and non-polar on the other, this allows the soap molecules to create a “cage” where the non-polar side is attracted to the non-polar dirt/oils, while the polar side is attracted to the water, which is a polar molecule. The attractive interactions are called intermolecular forces (more can be read here).
As you can see, soap is quite interesting in the way that we wouldn’t first expect to think of using oils to help clean off oil, and hopefully you’ll have something to think about when you’re washing yourself off with your favourite shea butter soap bar infused with essential oils.
More information on the soap making process can be found here:
Make Your Own Soap! Part 1: The Chemistry Behind Soap Making
http://www.chemistryexplained.com/Ru-Sp/Soap.html
– Nathan Yan

2 responses to “Soap Chemistry: Cleaning Ourselves with Fat”